Ab initio single-point energy computations at the CCSD(T)/aug-cc-pVTZ level have been carried out on nine molecules possessing intramolecular π-type hydrogen bonding. These computations were done using the calculated geometrical structures from CCSD/cc-pVTZ optimizations. The outcome has been analyzed together with previous spectroscopic and theoretical work. Results are presented for 3-cyclopenten-1-ol, 2-indanol, 2-cylopenten-1-ol, 2-cyclohexen-1-ol, 3-cyclohexen-1-ol, and 2-cyclopropen-1-ol with O–H∙∙∙π(C=C) bonding. Results for 3-cyclopenten-1-amine and 2-cyclopropen1-amine with N–H∙∙∙π(C=C) bonding and for 2-cyclopropen-1-thiol with S–H∙∙∙π(C=C) bonding are also discussed. For each molecule, the conformer with a weak intramolecular π-type hydrogen bond was found to be the global minimum, with the exception of 2-cyclopropen-1-thiol. In this case, the S– H∙∙∙π bonded conformer is higher in energy, indicating a weaker interaction. The hydrogen bonded conformers for the cyclic alcohols typically have conformational energies about 250 to 350 cm−1 lower than the other conformers. For the amines the lowering is about half of that. The infrared and Raman spectra for several molecules in the O-H stretching region show hydrogen bonded conformers to be at the lowest frequencies. The calculated potential energy surfaces for six of the molecules are also presented.
- Open Access
- Article
Ab initio Quantum-Chemical Calculations and Spectroscopic Studies of the Intramolecular π-Type Hydrogen Bonding in Small Cyclic Molecules
Author Information
Received: 17 Sep 2025 | Revised: 11 Oct 2025 | Accepted: 14 Oct 2025 | Published: 20 Oct 2025
Abstract
Graphical Abstract
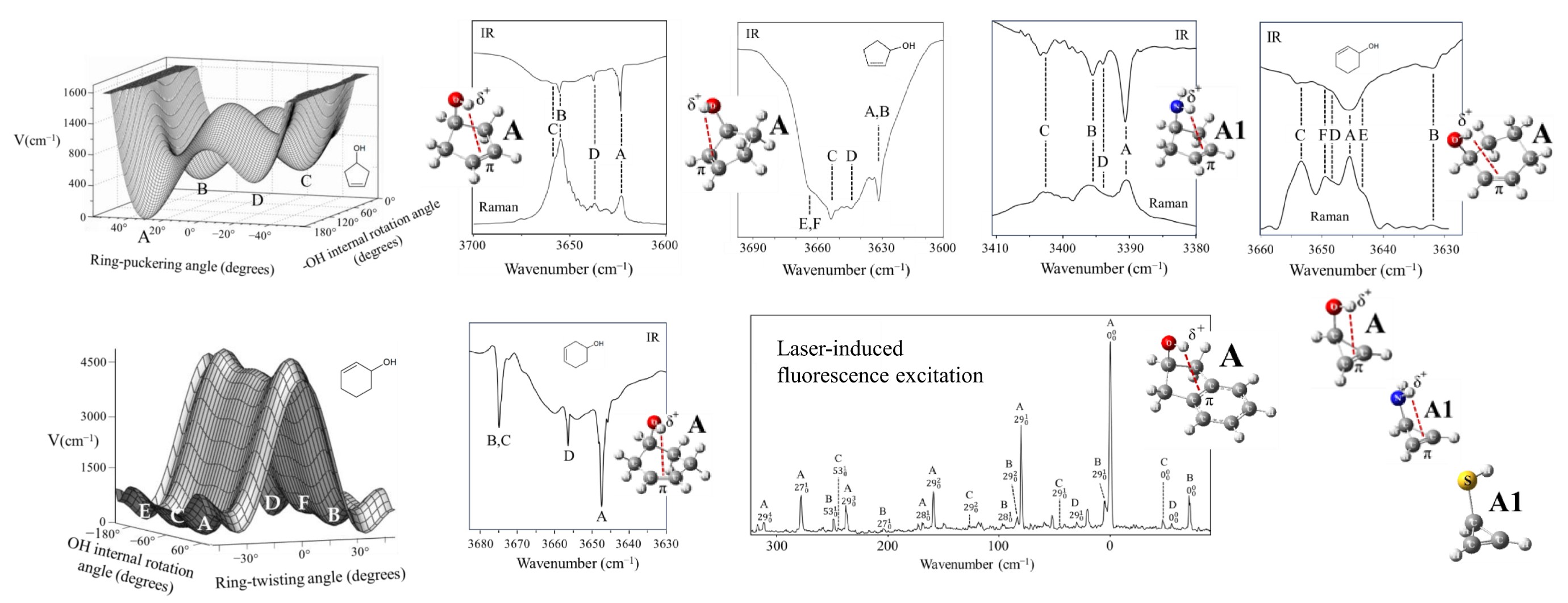
Keywords
π-type intramolecular hydrogen bonding | Raman spectroscopy | infrared spectroscopy | conformations | potential energy surface | CCSD(T)/aug-cc-pVTZ
References
- 1.Jeffrey, G.A.; Saenger, W. Hydrogen Bonding in Biological Structures, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1991.
- 2.Jeffrey, G.A. An Introduction to Hydrogen Bonding; Oxford University Press: New York, NY, USA, 1997.
- 3.Kojić-Prodić, B.K.; Molčanov, K. The Nature of Hydrogen Bond: New Insights into Old Theories. Acta Chim. Slov. 2008, 55, 692–708.
- 4.Zhyganiuk, I.V.; Malomuzh, M.P. Physical Nature of Hydrogen Bond. Ukr. J. Phys. 2015, 60, 960–974.
- 5.Grabowski, S.J. (Ed.) Hydrogen Bonding—New Insights, 1st ed.; Springer: Dordrecht, The Netherlands, 2006.
- 6.Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology, 1st ed.; Oxford University Press: New York, NY, USA, 1998; Reprinted 2001.
- 7.Gilli, G.; Gilli, P. The Nature of the Hydrogen Bond–Outline of a Comprehensive Hydrogen Bond Theory, 1st ed.; Oxford University Press: New York, NY, USA, 2009.
- 8.Steiner, T.; Koellner, G. Hydrogen Bonds with π-Acceptors in Proteins: Frequencies and Role in Stabilizing Local 3D Structures. J. Mol. Biol. 2001, 305, 535–557.
- 9.Meyer, E.A.; Castellano, R.K.; Diederich, F. Interactions with Aromatic Rings in Chemical and Biological Recognition. Angew. Chem. Int. Ed. Engl. 2003, 42, 1210–1250.
- 10.Liaquat, H.; Imran, M.; Saddique, Z.; et al. Exploring the Versatility of Hydrogen-Bonded Organic Frameworks: Advances in Design, Stability, and Multifunctional Applications. J. Mol. Struct. 2025, 1321, 140221.
- 11.Elewa, A.M. Hydrogen-Bonded Organic Frameworks (HOFs) from Design to Environmental Application. J. Ind. Eng. Chem. 2025, 145, 169–190.
- 12.Takasashi, O.; Kohno, Y.; Nishio, M. Relevance of Weak Hydrogen Bonds in the Conformation of Organic Compounds and Bioconjugates: Evidence from Recent Experimental Data and High-Level ab Initio MO Calculations. Chem. Rev. 2010, 110, 6049–6076.
- 13.Salonen, L.M.; Ellermann, M.; Diederich, F. Aromatic Rings in Chemical and Biological Recognition: Energetics and Structures. Angew. Chem. Int. Ed. Engl. 2011, 50, 4808–4842.
- 14.Arkas, M.; Kitsou, O.; Gkouma, A.; et al. The Role of Hydrogen Bonds in the Mesomorphic Behaviour of Supramolecular Assemblies Organized in Dendritic Architectures. Liq. Crys. Rev. 2019, 7, 60–105.
- 15.Bu, R.; Xiong, Y.; Wei, X.; et al. Hydrogen Bonding in CHON-Containing Energetic Crystals: A Review. Cryst. Growth Des. 2019, 19, 5981−5997.
- 16.Juhaś, M.; Zitko, J. Molecular Interactions of Pyrazine-Based Compounds to Proteins. J. Med. Chem. 2020, 63, 8901−8916.
- 17.Liu, A.; Zhou, Y.; Yuan, L. Hydrogen-Bonded Aromatic Amide Macrocycles: Synthesis, Properties and Functions. Org. Biomol. Chem. 2022, 20, 9023–9051.
- 18.Samuel, H.S.; Nweke-Maraizu, U.; Etim, E.E. Understanding Intermolecular and Intramolecular Hydrogen Bonds: Spectroscopic and Computational Approaches. J. Chem. Rev. 2023, 5, 439–465.
- 19.Haque, A.; Alenezi, K.M.; Khan, M.S.; et al. Non-Covalent Interactions (NCIs) In π-Conjugated Functional Materials: Advances and Perspectives. Chem. Soc. Rev. 2023, 52, 454–472.
- 20.Lin, L.; Jones, T.W.; Yang, T.C.J.; et al. Hydrogen Bonding in Perovskite Solar Cells. Matter 2024, 7, 38–58.
- 21.Grabowski, S.J. Hydrogen Bond Types Which do not fit Accepted Definitions. Chem. Commun. 2024, 60, 6239–6255.
- 22.Rao, C.N.R.; Murthy, A.S.N. Spectroscopic Studies of Hydrogen Bonding. In Developments in Applied Spectroscopy; Grove, E.L., Perkins, A.J., Eds.; Springer: Boston, MA, USA, 1970; Volume 7b, pp. 54–88.
- 23.Møllendal, H. Recent Gas·Phase Studies of Intramolecular Hydrogen Bonding. In Structures and Conformations of Non-Rigid Molecules, NATO ASI Series, Series C: Mathematical and Physical Science; Laane, J., Dakkouri, M., van der Veken, B., et al., Eds.; Springer Science + Business Media, B.V.: Dordrecht, The Netherlands, 1993; Volume 410, pp. 277–301.
- 24.Kovács, A.; Szabó, A.; Hargittai, I. Structural Characteristics of Intramolecular Hydrogen Bonding in Benzene Derivatives. Acc. Chem. Res. 2002, 35, 887–894.
- 25.Lyssenko, K.A.; Antipin, M.Y. The Nature and Energy Characteristics of Intramolecular Hydrogen Bonds in crystals. Russ. Chem. Bull. 2006, 55, 1–15.
- 26.Sobczyk, L.; Chudoba, D.; Tolstoy, P.M.; et al. Some Brief Notes on Theoretical and Experimental Investigations of Intramolecular Hydrogen Bonding. Molecules 2016, 21, 1657.
- 27.Hansen, P.E.; Spanget-Larsen, J. NMR and IR Investigations of Strong Intramolecular Hydrogen Bonds. Molecules 2017, 22, 552.
- 28.Caron, G.; Vallaro, M.; Ermondi, G. High Throughput Methods to Measure the Propensity of Compounds to form Intramolecular Hydrogen Bonding. Med. Chem. Commun. 2017, 8, 1143–1151.
- 29.Jezierska, A.; Tolstoy, P.M.; Panek, J.J.; et al. Intramolecular Hydrogen Bonds in Selected Aromatic Compounds: Recent Developments. Catalysts 2019, 9, 909.
- 30.Caron, G.; Kihlberg, J.; Ermondi, G. Intramolecular Hydrogen Bonding: An Opportunity for Improved Design in Medicinal Chemistry. Med. Res. Rev. 2019, 39, 1707–1729.
- 31.Deshmukh, M.M.; Gadre, S.R. Molecular Tailoring Approach for the Estimation of Intramolecular Hydrogen Bond Energy. Molecules 2021, 26, 2928.
- 32.Hansen, P.E. A Spectroscopic Overview of Intramolecular Hydrogen Bonds of NH…O, S, N Type. Molecules 2021, 26, 2409.
- 33.Hakobyan, R.M.; Shahkhatuni, A.G.; Polynski, M.V.; et al. The Role of the Nitro Group on the Formation of Intramolecular Hydrogen Bond in the Methyl Esters of 1-Vinyl-nitro-pyrazolecarboxylic Acids. J. Mol. Struct. 2025, 1322, 140350.
- 34.Rouquet, E.; Dupont, J.; Vincent, J.; et al. The Role of Intramolecular Hydrogen Bonding in Photoelectron Circular Dichroism: The Diastereoisomers of 1-Amino-2-Indanol. Phys. Chem. Chem. Phys. 2025, 27, 2739–2748.
- 35.Baker, A.W.; Shulgin, A.T. Intramolecular Hydrogen Bonds to π-Electrons and Other Weakly Basic Group. J. Am. Chem. Soc. 1958, 80, 5358–5363.
- 36.Morokuma, K.; Wipff, G. Theoretical Evidence for Intramolecular Hydrogen Bonding in 7-Norbornenol. Chem. Phys. Lett. 1980, 74, 400–403.
- 37.Smith, Z.; Carballo, N.; Wilson, E.B.; et al. Conformations, Possible Hydrogen Bonding, and Microwave Spectrum of 3-Buten-2-ol. J. Am. Chem. Soc. 1985, 107, 1951–1957.
- 38.Bakke, J.M.; Schie, A.M.; Skjetne, T. Conformation of Allylic Alcohols and Intramolecular Hydrogen Bonding. Acta Chem. Scand. B 1986, 40, 703–710.
- 39.Marstokk, K.-M.; Møllendal, H. Microwave Spectrum, Conformation and Intramolecular Hydrogen Bonding of 1,4-Pentadien-3-ol. Acta Chem. Scand. 1990, 44, 18–22.
- 40.Marstokk, K.-M.; Møllendal, H.; Samdal, S. Microwave Spectrum, Conformation and Intramolecular Hydrogen Bonding of 1-Mercapto-2-propanol. Acta Chem. Scand. 1990, 44, 339–345.
- 41.Melandri, S.; Favero, P.G.; Caminati, W. Detection of the syn Conformer of Ally1 Alcohol by Free Jet Microwave Spectroscopy. Chem. Phys. Lett. 1994, 223, 541–545.
- 42.Bräse, S.; Klæboe, P.; Marstokk, K.-M.; et al. Conformational Properties of 2-Cyclopropylideneethanol as Studied by Microwave, Infrared and Raman Spectroscopy and by Ab Initio Computations. Acta Chem. Scand. 1998, 52, 1122–1136.
- 43.Leonov, A.; Marstokk, K.-M.; de Meijere, A.; et al. Microwave Spectrum, Conformational Equilibrium, Intramolecular Hydrogen Bonding, Tunneling and Quantum Chemical Calculations for 1-Ethenylcyclopropan-1-ol. J. Phys. Chem. A 2000, 104, 4421–4428.
- 44.Rademacher, P.; Khelashvili, L.; Kowski, K. Spectroscopic and Theoretical Studies on Intramolecular OH–π Hydrogen Bonding in 4-Substituted 2-Allylphenols. Org. Biomol. Chem. 2005, 3, 2620–2625.
- 45.Isozaki, T.; Tsutsumi, Y.; Suzuki, T.; et al. Direct Evidence for Weak Intramolecular O–Hˑˑˑπ Hydrogen Bonding in 1-Hydroxytetralin. Chem. Phys. Lett. 2010, 495, 175–178.
- 46.Miller, B.J.; Lane, J.R.; Kjaergaard, H.G. Intramolecular OH⋯π Interactions in Alkenols and Alkynols. Phys. Chem. Chem. Phys. 2011, 13, 14183–14193.
- 47.Mackeprang, K.; Schrøder, S.D.; Kjaergaard, H.G. Weak Intramolecular OHˑˑˑπ Hydrogen Bonding in Methallyl- and Allyl-Carbinol. Chem. Phys. Lett. 2013, 582, 31–37.
- 48.Das, P.; Das, P.K.; Arunan, E. Conformational Stability and Intramolecular Hydrogen Bonding in 1,2-Ethanediol and 1,4-Butanediol. J. Phys. Chem. A 2015, 119, 3710−3720.
- 49.Al-Saadi, A.A.; Ocola, E.J.; Laane, J. Intramolecular π-Type Hydrogen Bonding and Conformations of 3-Cyclopenten-1-ol. 1. Theoretical Calculations. J. Phys. Chem. A 2010, 114, 7453–7456.
- 50.Ocola, E.J.; Al-Saadi, A.A.; Mlynek, C.; et al. Intramolecular π-Type Hydrogen Bonding and Conformations of 3-Cyclopenten-1-ol. 2. Infrared and Raman Spectral Studies at High Temperatures. J. Phys. Chem. A 2010, 114, 7457–7461.
- 51.Ocola, E.J. Vibrational and Theoretical Investigations of Molecular Conformations and Intramolecular π-Type Hydrogen Bonding. Ph. D. Thesis, Texas A & M University, College Station, TX, USA, 2011.
- 52.Al-Saadi, A.A.; Wagner, M.; Laane, J. Spectroscopic and Computational Studies of the Intramolecular Hydrogen Bonding of 2-Indanol. J. Phys. Chem. A 2006, 110, 12292–12297.
- 53.Ocola, E.J.; Laane, J. Spectroscopic and Theoretical Study of the Intramolecular π-Type Hydrogen Bonding and Conformations of 2-Cyclopenten-1-ol. Molecules 2021, 26, 1106.
- 54.Ocola, E.J.; Laane, J. Spectroscopic and Theoretical Study of the Intramolecular π-Type Hydrogen Bonding and Conformations of 2-Cyclohexen-1-ol. J. Phys Chem. A 2016, 120, 74–80.
- 55.Ocola, E.J.; Laane, J. Spectroscopic and Theoretical Study of the Intramolecular π-Type Hydrogen Bonding and Conformations of 3-Cyclohexen-1-ol. J. Mol. Spectrosc. 2022, 387, 111663.
- 56.Laane, J.; Ocola, E.J.; Chun, H.J. Vibrational Potential Energy Surfaces in Ground and Excited Electronic States. In Frontiers and Advances in Molecular Spectroscopy, 1st ed.; Laane, J., Ed.; Elsevier Publishing: Amsterdam, The Netherlands, 2017; Chapter 4, pp. 101–142.
- 57.Ocola, E.J.; Laane, J. Theoretical Investigation of Intramolecular π-Type Hydrogen Bonding and Internal Rotation of 2-Cyclopropen-1-ol, 2-Cyclopropen-1-thiol and 2-Cyclopropen-1-amine. Mol. Phys. 2018, 17, 1404–1412.
- 58.Varfolomeeva, V.V.; Terentev, A.V. Weak Intramolecular and Intermolecular Hydrogen Bonding of Benzyl Alcohol, 2-Phenylethanol and 2-Phenylethylamine in the Adsorption on Graphitized Thermal Carbon Black. Phys. Chem. Chem. Phys., 2015, 17, 24282–24293.
- 59.Ocola, E.J.; Laane, J. Spectroscopic and Theoretical Study of the Intramolecular π-Type Hydrogen Bonding and Conformations of 3-Cyclopentene-1-amine. J. Phys. Chem. A 2020, 124, 5907–5916.
- 60.Marstokk, K.-M.; Møllendal, H. Microwave Spectrum, Conformation and Internal Hydrogen Bonding in N-Methylallylamine. Acta Chem. Scand. A 1986, 40, 615–621.
- 61.Marstokk, K.-M.; Møllendal, H. Microwave Spectra and Weak Intramolecular Hydrogen Bonding in 3-Butene-1-Thiol And N-Methylallylamine. In Structure and Dynamics of Weakly Bound Molecular Complexes, NATO ASI Series, Series C: Mathematical and Physical Science; Weber, A., Ed.; Springer Science + Business Media, B.V.: Dordrecht, The Netherlands, 1993; Volume 212, pp. 57–68.
- 62.Marstokk, K.-M.; Møllendal, H. Microwave Spectrum, Conformational Equilibria and Intramolecular Hydrogen Bonding of 1-Amino-3-butene. Acta Chem. Scand. A 1988, 42, 374–390.
- 63.Marstokk, K.-M.; de Meijere, A.; Møllendal, H.; et al. Microwave Spectrum, Conformation, Dipole Moment and Quantum Chemical Calculations of 1-Amino-1-ethenylcyclopropane. J. Phys. Chem. A 2000, 104, 2897–2901.
- 64.Sastry, K.V.L.N.; Dass, S.C.; Brooks, W.V.F.; et al. Microwave Spectrum, Dipole Moment, and Molecular Structure of Allyl Mercaptan. J. Mol. Spect. 1969, 31, 54–65.
- 65.Marstokk, K.-M.; Møllendal, H. Microwave Spectrum, Conformational Equilibria, Intramolecular Hydrogen Bonding and Centrifugal Distortion of 3-Butene-1-thiol. Acta Chem. Scand. 1986, 40, 402–411.
- 66.Laane, J. One-dimensional potential energy functions in vibrational spectroscopy. Q. Rev. Chem. Soc. 1971, 25, 533–552.
- 67.Laane, J. Determination of Vibrational Potential Energy Surfaces from Raman and Infrared Spectra. Pure Appl. Chem. 1987, 59, 1307–1326.
- 68.Laane, J. Vibrational Potential Energy Surfaces of Non-Rigid Molecules in Ground and Excited Electronic States. In Structures and Conformations of Non-Rigid Molecules, NATO ASI Series, Series C: Mathematical and Physical Science; Laane, J., Dakkouri, M., van der Veken, B., et al., Eds.; Springer Science + Business Media B.V.: Dordrecht, The Netherlands, 1993; Volume 410, 65-98.
- 69.Laane, J. Vibrational Potential Energy Surfaces and Conformations of Molecules in Ground and Excited Electronic States. Annu. Rev. Phys. Chem. 1994, 45, 179–211.
- 70.Laane, J. Spectroscopic Determination of Ground and Excited State Vibrational Potential Energy Surfaces. Int. Rev. Phys. Chem. 1999, 18, 301–341.
- 71.Laane, J. Vibrational Potential Energy Surfaces of Non-Rigid Molecules in Exited Electronic States. In Structure and Dynamics of Electronic Excited States; Laane, J., Takahashi, H., Bandrauk, A., Eds.; Springer: Berlin, Germany, 1999; pp. 3–35.
- 72.Laane, J. Experimental Determination of Vibrational Potential Energy Surfaces and Molecular Structures in Electronic Excited States. J. Chem. Phys. A 2000, 104, 7715–7733.
- 73.Laane, J. Vibrational Potential Energy Surfaces in Electronic Excited States. In Frontiers of Molecular Spectroscopy, 1st ed.; Laane, J., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2009; pp. 63–132.
- 74.Ocola, E.J.; Laane, J. Beyond the Harmonic Oscillator; Highlights of Selected Studies of Vibrational Potential Energy Functions. Molecules 2025, 30, 1492.
- 75.Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; et al. Gaussian 16 (Revision B.01); Gaussian Inc.: Wallingford, CT, USA, 2016.
- 76.Dennington, R.D., II; Keith, T.A.; Millam, J.M. GaussView (6.1.1), Graphical Interface; Semichem Inc.: Shawnee, KS, USA, 2000–2019.
- 77.Maple (2015, 2019.2); Maplesoft, a Division of Waterloo Maple Inc.: Waterloo, ON, Canada, 2015.
- 78.Haller, K.; Chiang, W.-Y.; del Rosario, A.; et al. High-Temperature Vapor-Phase Raman Spectra and Assignment of the Low-Frequency Modes of trans-Stilbene and 4-Methoxy-trans-Stilbene. J. Mol. Struct. 1996, 379, 19−23.
- 79.Laane, J.; Haller, K.; Sakurai, S.; et al. Raman Spectroscopy of Vapors at Elevated Temperatures. J. Mol. Struct. 2003, 650, 57−68.
How to Cite
Ocola, E. J.; Laane, J. Ab initio Quantum-Chemical Calculations and Spectroscopic Studies of the Intramolecular π-Type Hydrogen Bonding in Small Cyclic Molecules. Photochemistry and Spectroscopy 2025, 1 (1), 2. https://doi.org/10.53941/ps.2025.100002.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


