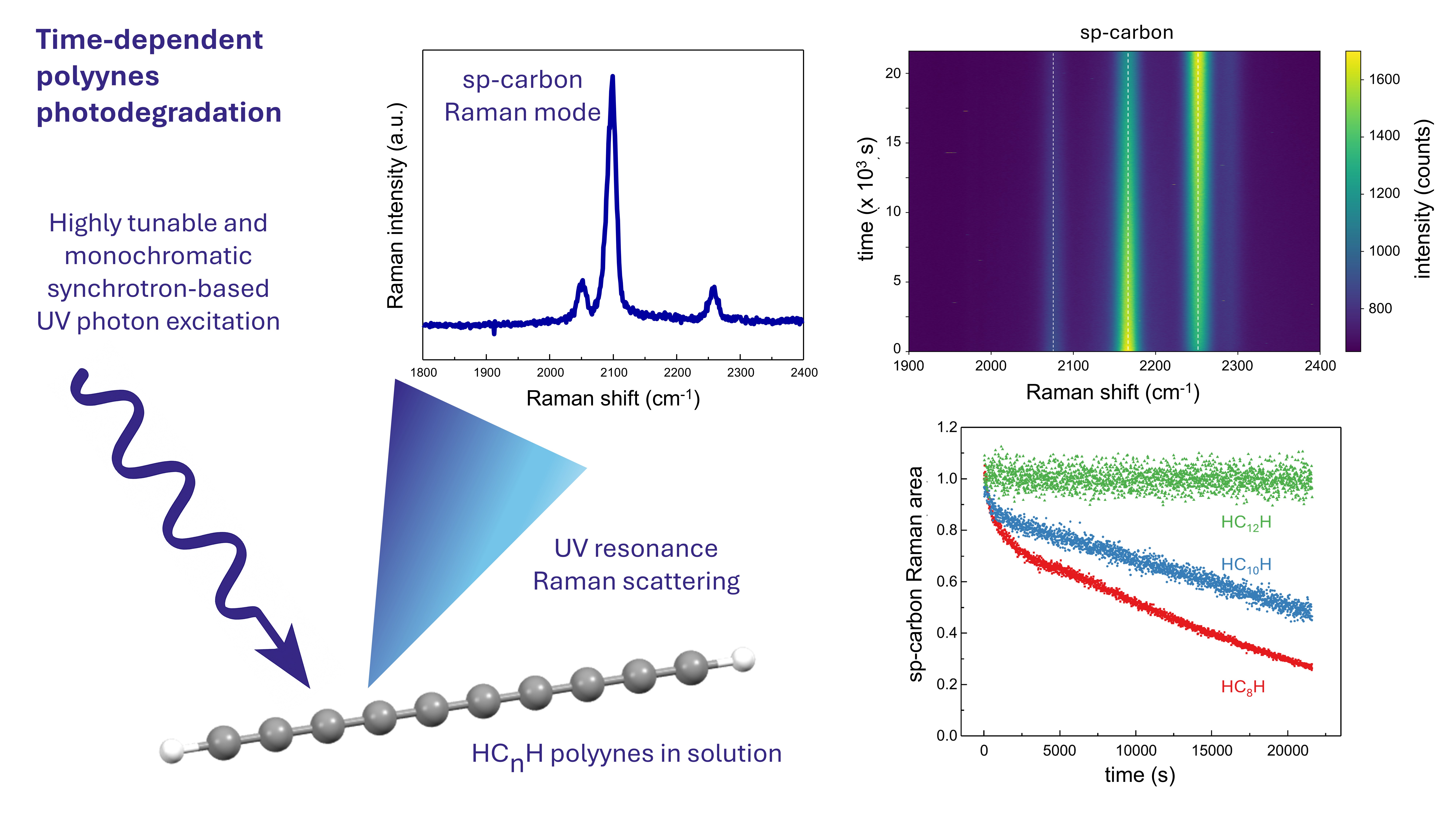
Carbon atomic wires (CAWs), linear one-dimensional carbon nanostructures, are attracting increasing attention in materials science due to their remarkable electrical, mechanical, optical, and transport properties, which make them promising candidates for being the next generation supercapacitors, batteries, hydrogen storage, organic semiconductors, and active optical elements. However, their intrinsic instability currently hinders their practical implementation. Previous studies have shown that CAWs mainly degrade through crosslinking interactions, exposure to high temperatures, hydrogenation and oxidation. Furthermore, a clear relationship between the wire structure, solvent polarity, and stability has been observed, with longer wires and more polar solvents reducing CAWs stability, while terminal groups strongly influence the degradation processes. Despite this, the photodegradation kinetics has not yet been fully and systematically investigated. Gaining such understanding is of fundamental importance for the rational design of CAWs-based materials for optoelectronic applications where light exposure is inevitable. In this work, we introduce a synchrotron-based approach that enables precise photoexcitation of CAWs with different chemical structures, tuned in resonance with their characteristic absorption vibronic peaks in the UV. This UV resonance Raman approach allows real-time monitoring of photodegradation directly through the time evolution of Raman spectra of each wire. We compare the photostability in different environments (i.e., acetonitrile, water, and aqueous colloidal silver nanoparticle dispersions), focusing on the role of two key structural parameters—sp-carbon chain length and terminal functional groups—in controlling the stabilization of these systems.
- Open Access
- Article
Tunable UV Photoexcitation of Carbon Atomic Wires: Investigating the Roles of Chain Length, Termination Groups, and Environment
- Simone Melesi 1,
- Pietro Marabotti 1,2,
- Barbara Rossi 3,
- Valeria Russo 1,
- Andrea Li Bassi 1,
- Chiara Bertarelli 4,
- Carlo E. Bottani 1,
- Carlo S. Casari 1,*
Author Information
Received: 31 Oct 2025 | Revised: 01 Dec 2025 | Accepted: 02 Dec 2025 | Published: 20 Jan 2026
Abstract
Graphical Abstract
Keywords
carbon | polyynes | photodegradation | nanoparticles | UV resonance Raman spectroscopy | synchrotron
References
- 1.
Casari, C.S.; Tommasini, M.; Tykwinski, R.R.; et al. Carbon-atom wires: 1-D systems with tunable properties. Nanoscale 2016, 8, 4414–4435.
- 2.
Casari, C.S.; Milani, A. Carbyne: From the elusive allotrope to stable carbon atom wires. MRS Commun. 2018, 8, 207–219.
- 3.
Ghosh, S.; Righi, M.; Melesi, S.; et al. Cumulenic sp-Carbon atomic wires wrapped with polymer for supercapacitor application. Carbon 2025, 234, 119952. https://doi.org/10.1016/j.carbon.2024.119952.
- 4.
Bettini, L.G.; Della Foglia, F.; Piseri, P.; et al. Interfacial properties of a carbyne-rich nanostructured carbon thin film in ionic liquid. Nanotechnology 2016, 27, 115403.
- 5.
Zhang, D.; Yang, Z.; Li, X.; et al. Synthesis of an Sp-Carbon-Rich Carbonaceous Material Exhibiting Distinctive Anode Performance. Angew. Chem. Int. Ed. 2025, 64, e202514245. https://doi.org/10.1002/anie.202514245.
- 6.
Anikina, E.; Banerjee, A.; Beskachko, V.; et al. Li-decorated carbyne for hydrogen storage: Charge induced polarization and van’t Hoff hydrogen desorption temperature. Sustain. Energy Fuels 2020, 4, 691–699.
- 7.
Sorokin, P.B.; Lee, H.; Antipina, L.Y.; et al. Calcium-decorated carbyne networks as hydrogen storage media. Nano Lett. 2011, 11, 2660–2665.
- 8.
Scaccabarozzi, A.D.; Milani, A.; Peggiani, S.; et al. A field-effect transistor based on cumulenic sp-carbon atomic wires. J. Phys. Chem. Lett. 2020, 11, 1970–1974.
- 9.
Pecorario, S.; Scaccabarozzi, A.D.; Fazzi, D.; et al. Stable and Solution-Processable Cumulenic sp-Carbon Wires: A New Paradigm for Organic Electronics. Adv. Mater. 2022, 34, 2110468.
- 10.
Hu, F.; Zeng, C.; Long, R.; et al. Supermultiplexed optical imaging and barcoding with engineered polyynes. Nat. Methods 2018, 15, 194–200.
- 11.
Castiglioni, C.; Tommasini, M.; Zerbi, G. Raman spectroscopy of polyconjugated molecules and materials: Confinement effect in one and two dimensions. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2004, 362, 2425–2459. https://doi.org/10.1098/rsta.2004.1448.
- 12.
Tommasini, M.; Fazzi, D.; Milani, A.; et al. Intramolecular Vibrational Force Fields for Linear Carbon Chains through an Adaptative Linear Scaling Scheme. J. Phys. Chem. A 2007, 111, 11645–11651. https://doi.org/10.1021/jp0757006.
- 13.
Yang, S.; Kertesz, M.; Zólyomi, V.; et al. Application of a Novel Linear/Exponential Hybrid Force Field Scaling Scheme to the Longitudinal Raman Active Mode of Polyyne. J. Phys. Chem. A 2007, 111, 2434–2441. https://doi.org/10.1021/jp067866x.
- 14.
Yang, S.; Kertesz, M. Linear Cn Clusters: Are They Acetylenic or Cumulenic? J. Phys. Chem. A 2008, 112, 146–151. https://doi.org/10.1021/jp076805b.
- 15.
Melesi, S.; Pińkowski, P.; Pigulski, B.; et al. Probing the Stability of Halogenated Carbon Atomic Wires in Electrospun Nanofibers via Raman Spectroscopy. J. Phys. Chem. C 2025, 129, 12916–12926. https://doi.org/10.1021/acs.jpcc.5c02960.
- 16.
Melesi, S.; Marabotti, P.; Milani, A.; et al. Impact of Halogen Termination and Chain Length on π-Electron Conjugation and Vibrational Properties of Halogen-Terminated Polyynes. J. Phys. Chem. A 2024, 128, 2703–2716.
- 17.
Chalifoux, W.A.; Tykwinski, R.R. Synthesis of extended polyynes: Toward carbyne. Comptes Rendus Chim. 2009, 12, 341–358.
- 18.
Kudryavtsev, Y.U.P. Syntheses of carbyne and carbynoid structures. Carbyne Carbynoid Struct. 1999, 21, 39.
- 19.
Szafert, S.; Gladysz, J.A. Carbon in One Dimension: Structural Analysis of the Higher Conjugated Polyynes. Chem. Rev. 2003, 103, 4175–4206. https://doi.org/10.1021/cr030041o.
- 20.
Chalifoux, W.A.; Tykwinski, R.R. Synthesis of polyynes to model the sp-carbon allotrope carbyne. Nat. Chem. 2010, 2, 967–971. https://doi.org/10.1038/nchem.828.
- 21.
Tykwinski, R.R.; Chalifoux, W.; Eisler, S.; et al. Toward carbyne: Synthesis and stability of really long polyynes. Pure Appl. Chem. 2010, 82, 891–904.
- 22.
Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; et al. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. https://doi.org/10.1038/318162a0.
- 23.
Milani, P.; Iannotta, S. Cluster Beam Synthesis of Nanostructured Materials; Springer Science & Business Media: Berlin, Germany, 2012.
- 24.
Ravagnan, L.; Siviero, F.; Lenardi, C.; et al. Cluster-Beam Deposition and in situ Characterization of Carbyne-Rich Carbon Films. Phys. Rev. Lett. 2002, 89, 285506. https://doi.org/10.1103/PhysRevLett.89.285506.
- 25.
Casari, C.S.; Giannuzzi, C.S.; Russo, V. Carbon-atom wires produced by nanosecond pulsed laser deposition in a background gas. Carbon 2016, 104, 190–195.
- 26.
Matsutani, R.; Inoue, K.; Sanada, T.; et al. Preparation of long-chain polyynes of C28H2 and C30H2 by liquid-phase laser ablation. J. Photochem. Photobiol. A Chem. 2012, 240, 1–4.
- 27.
Marabotti, P.; Peggiani, S.; Vidale, A.; et al. Pulsed laser ablation in liquid of sp-carbon chains: Status and recent advances. Chin. Phys. B 2022, 31, 125202.
- 28.
Tsuji, M.; Kuboyama, S.; Matsuzaki, T.; et al. Formation of hydrogen-capped polyynes by laser ablation of C60 particles suspended in solution. Carbon 2003, 41, 2141–2148.
- 29.
Compagnini, G.; Mita, V.; Cataliotti, R.S.; et al. Short polyyne chains produced by pulsed laser ablation of graphite in water. Carbon 2007, 45, 2456–2458.
- 30.
Sato, Y.; Kodama, T.; Shiromaru, H.; et al. Synthesis of polyyne molecules from hexane by irradiation of intense femtosecond laser pulses. Carbon 2010, 48, 1673–1676. https://doi.org/10.1016/j.carbon.2009.12.036.
- 31.
Hu, A.; Sanderson, J.; Zaidi, A.A.; et al. Direct synthesis of polyyne molecules in acetone by dissociation using femtosecond laser irradiation. Carbon 2008, 46, 1823–1825. https://doi.org/10.1016/j.carbon.2008.07.036.
- 32.
Peggiani, S.; Marabotti, P.; Lotti, R.A.; et al. Solvent-dependent termination, size and stability in polyynes synthesized via laser ablation in liquids. Phys. Chem. Chem. Phys. 2020, 22, 26312–26321.
- 33.
Peggiani, S.; Facibeni, A.; Milani, A.; et al. In situ synthesis of polyynes in a polymer matrix via pulsed laser ablation in a liquid. Mater. Adv. 2020, 1, 2729–2736.
- 34.
Park, Y.E.; Shin, S.K.; Park, S.M. The physical effects on the formation of polyynes by laser ablation. Chem. Phys. Lett. 2013, 568–569, 112–116. https://doi.org/10.1016/j.cplett.2013.03.016.
- 35.
Matsutani, R.; Kakimoto, T.; Tanaka, H.; et al. Preparation of polyynes by liquid-phase laser ablation using different irradiation target materials and solvents. Carbon 2011, 49, 77–81. https://doi.org/10.1016/j.carbon.2010.08.044.
- 36.
Marabotti, P.; Peggiani, S.; Melesi, S.; et al. Exploring the Growth Dynamics of Size-Selected Carbon Atomic Wires with In Situ UV Resonance Raman Spectroscopy. Small 2024, 20, 2403054.
- 37.
Peggiani, S.; Facibeni, A.; Marabotti, P.; et al. A single liquid chromatography procedure to concentrate, separate and collect size-selected polyynes produced by pulsed laser ablation in water. Fuller. Nanotub. Carbon Nanostructures 2023, 31, 224–230. https://doi.org/10.1080/1536383X.2022.2137498.
- 38.
Wakabayashi, T.; Saikawa, M.; Wada, Y.; et al. Isotope scrambling in the formation of cyanopolyynes by laser ablation of carbon particles in liquid acetonitrile. Carbon 2012, 50, 47–56.
- 39.
Wakabayashi, T.; Szczepaniak, U.; Tanaka, K.; et al. Phosphorescence of hydrogen-capped linear polyyne molecules C8H2, C10H2 and C12H2 in solid hexane matrices at 20 K. Photochem 2022, 2, 181–201.
- 40.
Casari, C.S.; Bassi, A.L.; Ravagnan, L.; et al. Chemical and thermal stability of carbyne-like structures in cluster-assembled carbon films. Phys. Rev. B 2004, 69, 075422.
- 41.
Cataldo, F. Synthesis of polyynes in a submerged electric arc in organic solvents. Carbon 2004, 42, 129–142.
- 42.
Cataldo, F. Storage stability of polyynes and cyanopolyynes in solution and the effect of ammonia or hydrochloric acid. Fuller. Nanotub. Carbon Nanostructures 2007, 15, 155–166.
- 43.
Cataldo, F. Polyynes production in a solvent-submerged electric arc between graphite electrodes. III. Chemical reactivity and stability toward air, ozone, and light. Fuller. Nanotub. Carbon Nanostructures 2004, 12, 633–646. https://doi.org/10.1081/FST-200026951.
- 44.
Cataldo, F. Stability of polyynes in air and their degradation by ozonolysis. Polym. Degrad. Stab. 2006, 91, 317–323.
- 45.
Cui, W.; Saito, T.; Ayala, P.; et al. Oxidation stability of confined linear carbon chains, carbon nanotubes, and graphene nanoribbons as 1D nanocarbons. Nanoscale 2019, 11, 15253–15258. https://doi.org/10.1039/C9NR04924J.
- 46.
Rivelino, R.; Santos, R.B.D.; de Brito Mota, F.; et al. Conformational effects on structure, electron states, and Raman scattering properties of linear carbon chains terminated by graphene-like pieces. J. Phys. Chem. C 2010, 114, 16367–16372.
- 47.
Cataldo, F.; Ursini, O.; Milani, A.; et al. One-pot synthesis and characterization of polyynes end-capped by biphenyl groups (α,ω-biphenylpolyynes). Carbon 2018, 126, 232–240. https://doi.org/10.1016/j.carbon.2017.09.098.
- 48.
Arora, A.; Baksi, S.D.; Weisbach, N.; et al. Monodisperse Molecular Models for the sp Carbon Allotrope Carbyne; Syntheses, Structures, and Properties of Diplatinum Polyynediyl Complexes with PtC20Pt to PtC52Pt Linkages. ACS Cent. Sci. 2023, 9, 2225–2240.
- 49.
Pigulski, B.; Jarszak, A.; Szafert, S. Selective synthesis of iridium (iii) end-capped polyynes by oxidative addition of 1-iodopolyynes to Vaska’s complex. Dalton Trans. 2018, 47, 17046–17054.
- 50.
Gulia, N.; Pigulski, B.; Szafert, S. Palladium End-Capped Polyynes via Oxidative Addition of 1-Haloalkynes to Pd(PPh3)4. Organometallics 2015, 34, 673–682.
- 51.
Matsutani, R.; Ozaki, F.; Yamamoto, R.; et al. Preparation of polyynes up to C22H2 by liquid-phase laser ablation and their immobilization into SiO2 gel. Carbon 2009, 47, 1659–1663.
- 52.
Shi, L.; Rohringer, P.; Suenaga, K.; et al. Confined linear carbon chains as a route to bulk carbyne. Nat. Mater. 2016, 15, 634–639. https://doi.org/10.1038/nmat4617.
- 53.
Nishide, D.; Dohi, H.; Wakabayashi, T.; et al. Single-wall carbon nanotubes encaging linear chain C10H2 polyyne molecules inside. Chem. Phys. Lett. 2006, 428, 356–360. https://doi.org/10.1016/j.cplett.2006.07.016.
- 54.
Zhang, Y.; Zhao, J.; Fang, Y.; et al. Preparation of long linear carbon chain inside multi-walled carbon nanotubes by cooling enhanced hydrogen arc discharge method. Nanoscale 2018, 10, 17824–17833. https://doi.org/10.1039/C8NR05465G.
- 55.
Okada, S.; Fujii, M.; Hayashi, S. Immobilization of polyynes adsorbed on Ag nanoparticle aggregates into poly (vinyl alcohol) films. Carbon 2011, 49, 4704–4709.
- 56.
An, K.; Wei, G.; Qi, G.; et al. Stability improvement of C8H2 and C10H2 embedded in poly (vinyl alcohol) films with adsorption on gold nanoparticles. Chem. Phys. Lett. 2015, 637, 71–76.
- 57.
Casari, C.S.; Russo, V.; Bassi, A.L.; et al. Stabilization of linear carbon structures in a solid Ag nanoparticle assembly. Appl. Phys. Lett. 2007, 90, 013111.
- 58.
Kabaciński, P.; Marabotti, P.; Fazzi, D.; et al. Disclosing Early Excited State Relaxation Events in Prototypical Linear Carbon Chains. J. Am. Chem. Soc. 2023, 145, 18382–18390. https://doi.org/10.1021/jacs.3c04163.
- 59.
Cataldo, F.; Strazzulla, G.; Iglesias-Groth, S. UV photolysis of polyynes at λ = 254 nm and at λ > 222 nm. Int. J. Astrobiol. 2008, 7, 107–116.
- 60.
Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395.
- 61.
Amirjani, A.; Firouzi, F.; Haghshenas, D.F. Predicting the size of silver nanoparticles from their optical properties. Plasmonics 2020, 15, 1077–1082.
- 62.
D’Amico, F.; Saito, M.; Bencivenga, F.; et al. UV resonant Raman scattering facility at Elettra. Nucl. Instrum. Methods Phys. Res. A 2013, 703, 33–37. https://doi.org/10.1016/j.nima.2012.11.037.
- 63.
Inoue, K.; Matsutani, R.; Sanada, T.; et al. Preparation of long-chain polyynes of C24H2 and C26H2 by liquid-phase laser ablation in decalin. Carbon 2010, 48, 4209–4211. https://doi.org/10.1016/j.carbon.2010.07.020.
- 64.
Milani, A.; Tommasini, M.; Barbieri, V.; et al. Semiconductor-to-metal transition in carbon-atom wires driven by sp2 conjugated end groups. J. Phys. Chem. C 2017, 121, 10562–10570.
- 65.
Milani, A.; Barbieri, V.; Facibeni, A.; et al. Structure modulated charge transfer in carbon atomic wires. Sci. Rep. 2019, 9, 1648.
- 66.
Gao, Y.; Hou, Y.; Gamez, F.G.; et al. The loss of endgroup effects in long pyridyl-endcapped oligoynes on the way to carbyne. Nat. Chem. 2020, 12, 1143–1149.
- 67.
Milani, A.; Tommasini, M.; Russo, V.; et al. Raman spectroscopy as a tool to investigate the structure and electronic properties of carbon-atom wires. Beilstein J. Nanotechnol. 2015, 6, 480–491.
- 68.
Tabata, H.; Fujii, M.; Hayashi, S.; et al. Raman and surface-enhanced Raman scattering of a series of size-separated polyynes. Carbon 2006, 44, 3168–3176.
- 69.
Tommasini, M.; Milani, A.; Fazzi, D.; et al. π-Conjugation and End Group Effects in Long Cumulenes: Raman Spectroscopy and DFT Calculations. J. Phys. Chem. C 2014, 118, 26415–26425. https://doi.org/10.1021/jp509724d.
- 70.
Lee, S.; Heller, E.J. Time-dependent theory of Raman scattering. J. Chem. Phys. 1979, 71, 4777–4788. https://doi.org/10.1063/1.438316.
- 71.
Albrecht, A.C. On the Theory of Raman Intensities. J. Chem. Phys. 1961, 34, 1476–1484. https://doi.org/10.1063/1.1701032.
- 72.
Marabotti, P.; Tommasini, M.; Castiglioni, C.; et al. Electron-phonon coupling and vibrational properties of size-selected linear carbon chains by resonance Raman scattering. Nat. Commun. 2022, 13, 5052.
- 73.
Marabotti, P.; Tommasini, M.; Castiglioni, C.; et al. Synchrotron-based UV resonance Raman spectroscopy probes size confinement, termination effects, and anharmonicity of carbon atomic wires. Carbon 2024, 216, 118503. https://doi.org/10.1016/j.carbon.2023.118503.
- 74.
Asher, S.A. UV resonance Raman studies of molecular structure and dynamics. Annu. Rev. Phys. Chem. 1988, 39, 537–588.
- 75.
Ludwig, M.; Asher, S.A. Self-Absorption in Resonance Raman and Rayleigh Scattering: A Numerical Solution. Appl. Spectrosc. 1988, 42, 1458–1466. https://doi.org/10.1366/0003702884429670.
- 76.
Hong, Z.; Asher, S.A. Dependence of Raman and Resonance Raman Intensities on Sample Self-Absorption. Appl. Spectrosc. 2015, 69, 75–83. https://doi.org/10.1366/14-07531.
- 77.
Peggiani, S.; Marabotti, P.; Lotti, R.A.; et al. Solvent-dependent termination, size and stability in polyynes synthesized via laser ablation in liquid. Phys. Chem. Chem. Phys. 2020, 22, 26312–26321. https://doi.org/10.1039/D0CP04132G.
- 78.
Cataldo, F.; Ursini, O.; Angelini, G. Kinetics of polyynes formation with the submerged carbon arc. J. Electroanal. Chem. 2007, 602, 82–90.
- 79.
Ye, C.; Gray, V.; Mårtensson, J.; et al. Annihilation versus excimer formation by the triplet pair in triplet–triplet annihilation photon upconversion. J. Am. Chem. Soc. 2019, 141, 9578–9584.
- 80.
Hoche, J.; Schmitt, H.-C.; Humeniuk, A.; et al. The mechanism of excimer formation: An experimental and theoretical study on the pyrene dimer. Phys. Chem. Chem. Phys. 2017, 19, 25002–25015. https://doi.org/10.1039/C7CP03990E.
- 81.
Pigulski, B.; Gulia, N.; Szafert, S. Reactivity of Polyynes: Complex Molecules from Simple Carbon Rods. Eur. J. Org. Chem. 2019, 2019, 1420–1445. https://doi.org/10.1002/ejoc.201801350.
- 82.
Chalifoux, W.A.; Tykwinski, R.R. Oligoynes and polyynes. Can. J. Chem. 2025. https://doi.org/10.1139/cjc-2025-0069.
- 83.
Yosipof, A.; Basch, H.; Hoz, S. Nucleophilic and Electrophilic Reactions of Polyynes Catalyzed by an Electric Field: Toward Barcoding of Carbon Nanotubes Like Long Homogeneous Substrates. J. Phys. Chem. A 2013, 117, 5023–5027. https://doi.org/10.1021/jp402758u.
- 84.
Movsisyan, L.D.; Peeks, M.D.; Greetham, G.M.; et al. Photophysics of Threaded sp-Carbon Chains: The Polyyne is a Sink for Singlet and Triplet Excitation. J. Am. Chem. Soc. 2014, 136, 17996–18008. https://doi.org/10.1021/ja510663z.
- 85.
Walvoord, R.R.; Huynh, P.N.H.; Kozlowski, M.C. Quantification of Electrophilic Activation by Hydrogen-Bonding Organocatalysts. J. Am. Chem. Soc. 2014, 136, 16055–16065. https://doi.org/10.1021/ja5086244.
- 86.
Zelikoff, M.; Aschenbrand, L.M. Vacuum Ultraviolet Photochemistry. Part III. Acetylene a 1849 A. J. Chem. Phys. 1956, 24, 1034–1037. https://doi.org/10.1063/1.1742673.
- 87.
Glicker, S.; Okabe, H. Photochemistry of diacetylene. J. Phys. Chem. 1987, 91, 437–440.
- 88.
Cataldo, F. A study on ethylene and acetylene photoligomerization and photopolymerization. J. Photochem. Photobiol. A Chem. 1996, 99, 75–81. https://doi.org/10.1016/1010-6030(96)04356-0.
- 89.
Tang, B.; Zhao, J.; Xu, J.-F.; et al. Tuning the stability of organic radicals: From covalent approaches to non-covalent approaches. Chem. Sci. 2020, 11, 1192–1204. https://doi.org/10.1039/C9SC06143F.
- 90.
Velloth, A.; Kumar, P.; Butt, S.; et al. C–Centered Radicals: Generation, Detection, Stability and Perspectives. Asian J. Org. Chem. 2025, 14, e202400686. https://doi.org/10.1002/ajoc.202400686.
- 91.
Cataldo, F. Polyynes production in a solvent-submerged electric arc between graphite electrodes. III. chemical reactivity and stability toward air, ozone, and light. Fuller. Nanotub. Carbon Nanostructures 2004, 12, 633–646.
- 92.
McGivern, W.S.; Derecskei-Kovacs, A.; North, S.W.; et al. Computationally Efficient Methodology to Calculate C−H and C−X (X = F, Cl, and Br) Bond Dissociation Energies in Haloalkanes. J. Phys. Chem. A 2000, 104, 436–442. https://doi.org/10.1021/jp993275d.
- 93.
Cioslowski, J.; Liu, G.; Moncrieff, D. Thermochemistry of Homolytic C−C, C−H, and C−Cl Bond Dissociations in Polychloroethanes: Benchmark Electronic Structure Calculations. J. Am. Chem. Soc. 1997, 119, 11452–11457. https://doi.org/10.1021/ja971108q.
- 94.
Vollhardt, K.P.C.; Schore, N.E. Organic Chemistry: Structure and Function; Macmillan: London, UK, 2003.
- 95.
Kim, H.; Tarakeshwar, P.; Fujikado, N.M.; et al. Pseudocarbynes: Linear carbon chains stabilized by metal clusters. J. Phys. Chem. C 2020, 124, 19355–19361.
- 96.
Marabotti, P.; Peggiani, S.; Facibeni, A.; et al. In situ surface-enhanced Raman spectroscopy to investigate polyyne formation during pulsed laser ablation in liquid. Carbon 2022, 189, 219–229. https://doi.org/10.1016/j.carbon.2021.12.060.
- 97.
Milani, A.; Lucotti, A.; Russo, V.; et al. Charge transfer and vibrational structure of sp-hybridized carbon atomic wires probed by surface enhanced Raman spectroscopy. J. Phys. Chem. C 2011, 115, 12836–12843.
- 98.
Lucotti, A.; Casari, C.S.; Tommasini, M.; et al. sp Carbon chain interaction with silver nanoparticles probed by Surface Enhanced Raman Scattering. Chem. Phys. Lett. 2009, 478, 45–50. https://doi.org/10.1016/j.cplett.2009.06.030.
- 99.
Condorelli, M.; Speciale, A.; Cimino, F.; et al. Nano-hybrid Au@ LCCs systems displaying anti-inflammatory activity. Materials 2022, 15, 3701.
- 100.
Grasso, G.; D’Urso, L.; Messina, E.; et al. A mass spectrometry and surface enhanced Raman spectroscopy study of the interaction between linear carbon chains and noble metals. Carbon 2009, 47, 2611–2619.
- 101.
Zhu, H.; Gablech, E.; Gablech, I.; et al. The collective photothermal effect of silver nanoparticles probed by a microbolometer. Commun. Mater. 2024, 5, 66. https://doi.org/10.1038/s43246-024-00509-0.
- 102.
Anuthum, S.; Hasegawa, F.; Lertvachirapaiboon, C.; et al. Plasmonic photothermal properties of silver nanoparticle grating films. Phys. Chem. Chem. Phys. 2022, 24, 7060–7067. https://doi.org/10.1039/D1CP05893B.
- 103.
Huang, H.; Sivayoganathan, M.; Duley, W.W.; et al. Efficient localized heating of silver nanoparticles by low-fluence femtosecond laser pulses. Appl. Surf. Sci. 2015, 331, 392–398. https://doi.org/10.1016/j.apsusc.2015.01.086.
- 104.
Movsisyan, L.D.; Franz, M.; Hampel, F.; et al. Polyyne Rotaxanes: Stabilization by Encapsulation. J. Am. Chem. Soc. 2016, 138, 1366–1376. https://doi.org/10.1021/jacs.5b12049.

This work is licensed under a Creative Commons Attribution 4.0 International License.


