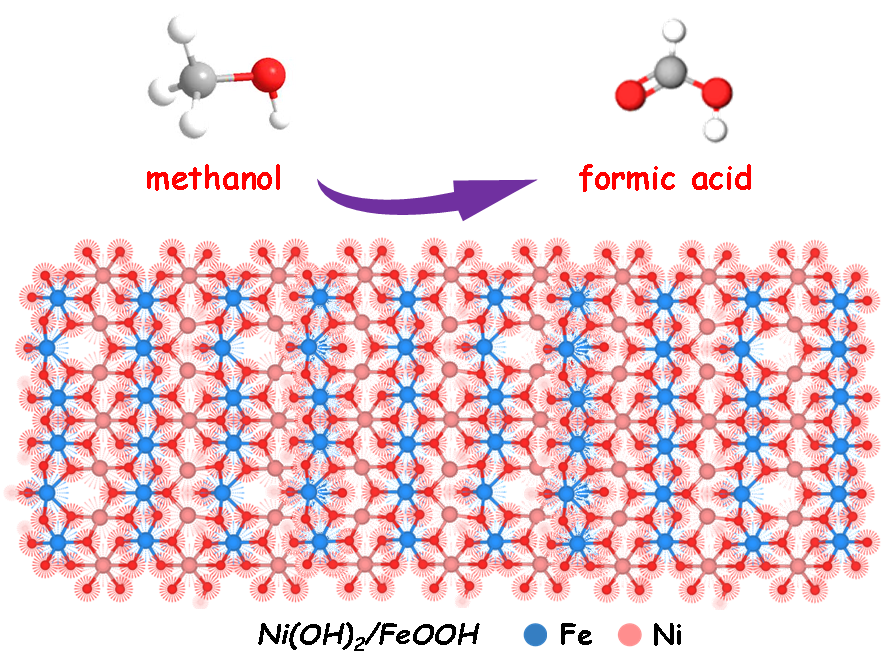
Electrocatalytic methanol oxidation reaction (MOR) holds significant value in the chemical industry, as it enables the treatment of methanol-containing wastewater and promotes hydrogen production from water. This study investigates a strategy based on tuning-composition of metal elements to optimize MOR performance, aiming to outperform the current cost-effective and efficient catalysts. To this end, nickel hydroxide and iron oxyhydroxide heterostructures were synthesized through a facile hydrothermal routine, and the catalytic performance of three different Ni/Fe ratios in MOR was examined in alkaline media. Among them, the material with equal Ni/Fe ratio exhibited the best catalytic activity, maintaining a high current density of ~66 mA cm−2 at 1.5 V vs. RHE in 1 M KOH electrolyte with 1 M methanol. Moreover, this developed electrode showed a Faradaic efficiency (FE) of 98.5% for formate production within a continuous 12 h test. Furthermore, density function theory (DFT) calculation was applied to unravel the methanol-to-formate conversion mechanism that was enhanced by the proper Ni/Fe ratio. These results demonstrate the high efficiency and selectivity of efficient methanol-to-formate conversion on NiFe-based materials, providing a promising a non-precious catalyst for electrocatalytic upgrading methanol to value-added formate.
- Open Access
- Article
Improved Methanol-to-Formate Electrocatalytic Reaction by Engineering of Nickel Hydroxide and Iron Oxyhydroxide Heterostructures
- Ning Jian 1, 2,
- Huan Ge 1, 2,
- Yi Ma 1, 2,
- Yong Zhang 1, 2,
- Luming Li 1, 2,
- Junfeng Liu 3,
- Jing Yu 4,
- Canhuang Li 4,
- Junshan Li 1, 2, *
Author Information
Received: 08 Mar 2025 | Revised: 21 Mar 2025 | Accepted: 25 Mar 2025 | Published: 27 Mar 2025
Abstract
Graphical Abstract
Keywords
electrocatalysis | methanol oxidation reaction | formate production | hydrogen evolution reaction | electrocatalytic upgrading
References
- 1.Campos-Seijo, B. Carbon-Neutrality Goals. C&EN Glob. Enterp. 2020, 98, 4. https://doi.org/10.1021/cen-09840-editorial.
- 2.Liu, C.; Li, F.; Lai-Peng, M.; Cheng, H.M. Advanced Materials for Energy Storage. Adv. Mater. 2010, 22, E28–E62. https://doi.org/10.1002/adma.200903328.
- 3.Yadav, V.G.; Yadav, G.D.; Patankar, S.C. The Production of Fuels and Chemicals in the New World: Critical Analysis of the Choice between Crude Oil and Biomass Vis-à-Vis Sustainability and the Environment. Clean Technol. Environ. Policy 2020, 22, 1757–1774. https://doi.org/10.1007/s10098-020-01945-5.
- 4.Turner, J.A. Sustainable Hydrogen Production. Science 2004, 305, 972–974. https://doi.org/10.1126/science.1103197.
- 5.Wang, P.; Zhang, R.; Wang, K.; Liu, Y.; Zhang, L.; Wang, X.; Li, H.; He, Y.; Liu, Z. Simultaneously Constructing Asymmetrically Coordinated Cobalt Single Atoms and Cobalt Nanoclusters via a Fresh Potassium Hydroxide Clipping Strategy toward Efficient Alkaline Oxygen Reduction Reaction. Energy Mater. Adv. 2023, 4, 0042. https://doi.org/10.34133/energymatadv.0042.
- 6.Moges, E.A.; Lakshmanan, K.; Chang, C.Y.; Liao, W.S.; Angerasa, F.T.; Dilebo, W.B.; Edao, H.G.; Tadele, K.T.; Alemayehu, D.D.; Bejena, B.D.; et al. Materials of Value-Added Electrolysis for Green Hydrogen Production. ACS Mater. Lett. 2024, 6, 4932–4954. https://doi.org/10.1021/acsmaterialslett.4c01173.
- 7.Moges, E.A.; Chang, C.Y.; Tsai, M.C.; Su, W.N.; Hwang, B.J. Electrocatalysts for Value-Added Electrolysis Coupled with Hydrogen Evolution. EES Catal. 2023, 1, 413–433. https://doi.org/10.1039/d3ey00017f.
- 8.McCrory, C.C.L.; Jung, S.; Ferrer, I.M.; Chatman, S.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking Hydrogen Evolving Reaction and Oxygen Evolving Reaction Electrocatalysts for Solar Water Splitting Devices. J. Am. Chem. Soc. 2015, 137, 4347–4357. https://doi.org/10.1021/ja510442p.
- 9.Stamenkovic, V.R.; Strmcnik, D.; Lopes, P.P.; Markovic, N.M. Energy and Fuels from Electrochemical Interfaces. Nat. Mater. 2016, 16, 57–69. https://doi.org/10.1038/nmat4738.
- 10.Xiang, K.; Wu, D.; Deng, X.; Li, M.; Chen, S.; Hao, P.; Guo, X.; Luo, J.L.; Fu, X.Z. Boosting H2 Generation Coupled with Selective Oxidation of Methanol into Value-Added Chemical over Cobalt Hydroxide@Hydroxysulfide Nanosheets Electrocatalysts. Adv. Funct. Mater. 2020, 30, 1909610. https://doi.org/10.1002/adfm.201909610.
- 11.Li, J.; Li, L.; Wang, J.; Cabot, A.; Zhu, Y. Boosting Hydrogen Evolution by Methanol Oxidation Reaction on Ni-Based Electrocatalysts: From Fundamental Electrochemistry to Perspectives. ACS Energy Lett. 2024, 9, 853–879. https://doi.org/10.1021/acsenergylett.3c02678.
- 12.Ren, J.; Zhang, Y.; Li, J.; Liu, J.; Hu, J.; Li, C.; Ke, Y.; Zhao, J.; Cabot, A.; Tang, B. Hydrothermal Nickel Selenides as Efficient Electrodes in Alkaline Media: Application to Supercapacitors and Methanol Oxidation Reaction. Dalt. Trans. 2024, 53, 18736–18744. https://doi.org/10.1039/D4DT02472A.
- 13.Ma, Y.; Li, L.; Tang, J.; Hu, Z.; Zhang, Y.; Ge, H.; Jian, N.; Zhao, J.; Cabot, A.; Li, J. Electrochemical PET Recycling to Formate through Ethylene Glycol Oxidation on Ni-Co-S Nanosheet Arrays. J. Mater. Chem. A 2024, 12, 33917–33925. https://doi.org/10.1039/d4ta07156e.
- 14.Zhang, Y.; Liu, R.; Ma, Y.; Jian, N.; Pan, H.; Liu, Y.; Deng, J.; Li, L.; Shao, Q.; Li, C.; et al. Nickel-Cobalt Oxide Nanoparticles as Superior Electrocatalysts for Enhanced Coupling Hydrogen Evolution and Selective Ethanol Oxidation Reaction. J. Mater. Chem. A 2024, 12, 17252–17259. https://doi.org/10.1039/d4ta03259d.
- 15.Kakati, N.; Maiti, J.; Lee, S.H.; Jee, S.H.; Viswanathan, B.; Yoon, Y.S. Anode Catalysts for Direct Methanol Fuel Cells in Acidic Media: Do We Have Any Alternative for Pt or Pt-Ru? Chem. Rev. 2014, 114, 12397–12429. https://doi.org/10.1021/cr400389f.
- 16.Wu, D.; Hao, J.; Song, Z.; Fu, X.Z.; Luo, J.L. All Roads Lead to Rome: An Energy-Saving Integrated Electrocatalytic CO2 Reduction System for Concurrent Value-Added Formate Production. Chem. Eng. J. 2020, 412, 127893. https://doi.org/10.1016/j.cej.2020.127893.
- 17.Liu, Y.P.; Zhao, S.F.; Guo, S.X.; Bond, A.M.; Zhang, J.; Zhu, G.; Hill, C.L.; Geletii, Y.V. Electrooxidation of Ethanol and Methanol Using the Molecular Catalyst [{Ru4O4(OH)2(H2O)4}(γ-SiW10O36)2]10−. J. Am. Chem. Soc. 2016, 138, 2617–2628. https://doi.org/10.1021/jacs.5b11408.
- 18.Zhang, Y.; Liu, R.; Ma, Y.; Jian, N.; Ge, H.; Pan, H.; Zhang, Y.; Zhang, C.; Liu, Y.; Deng, J.; et al. Surface Selenium Coating Promotes Selective Methanol-to-Formate Electrooxidation on Ni3Se4 Nanoparticles. Inorg. Chem. 2024, 63, 23328–23337. https://doi.org/10.1021/acs.inorgchem.4c03996.
- 19.Yi, Y.; Li, J.; Cui, C. Trimetallic FeCoNi Disulfide Nanosheets for CO2-Emission-Free Methanol Conversion. Chinese Chem. Lett. 2022, 33, 1006–1010. https://doi.org/10.1016/j.cclet.2021.07.005.
- 20.Liu, J.; Li, T.; Wang, Q.; Liu, H.; Wu, J.; Sui, Y.; Li, H.; Tang, P.; Wang, Y. Bifunctional PdMoPt Trimetallene Boosts Alcohol-Water Electrolysis. Chem. Sci. 2024, 15, 16660–16668. https://doi.org/10.1039/d4sc04764h.
- 21.Liu, H.; Li, T.; Wu, Z.; Xu, H.; Li, H.; Jing, R.; Wang, Y.; Liu, J. Integration of Phosphorus in PdCr Metallene for Enhanced CO-Tolerant Alcohol Electrooxidation. Inorg. Chem. 2024, 64, 123–132. https://doi.org/10.1021/acs.inorgchem.4c04334.
- 22.Liu, J.; Liu, H.; Wang, Q.; Li, T.; Yang, T.; Zhang, W.; Xu, H.; Li, H.; Qi, X.; Wang, Y.; et al. Phosphorus Doped PdMo Bimetallene as a Superior Bifunctional Fuel Cell Electrocatalyst. Chem. Eng. J. 2024, 486, 150258. https://doi.org/10.1016/j.cej.2024.150258.
- 23.Liu, J.; Wang, Q.; Li, T.; Wang, Y.; Li, H.; Cabot, A. PdMoSb Trimetallene as High-Performance Alcohol Oxidation Electrocatalyst. Nano Res. 2023, 16, 2041–2048. https://doi.org/10.1007/s12274-022-4873-8.
- 24.Zhou, J.; Yuan, L.; Wang, J.; Song, L.; You, Y.; Zhou, R.; Zhang, J.; Xu, J. Combinational Modulations of NiSe2 Nanodendrites by Phase Engineering and Iron-Doping towards an Efficient Oxygen Evolution Reaction. J. Mater. Chem. A 2020, 8, 8113–8120. https://doi.org/10.1039/d0ta00860e.
- 25.Chen, D.; Minteer, S.D. Mechanistic Study of Nickel Based Catalysts for Oxygen Evolution and Methanol Oxidation in Alkaline Medium. J. Power Sources 2015, 284, 27–37. https://doi.org/10.1016/j.jpowsour.2015.02.143.
- 26.Zhang, M.; Zhu, J.; Wan, R.; Liu, B.; Zhang, D.; Zhang, C.; Wang, J.; Niu, J. Synergistic Effect of Nickel Oxyhydroxide and Tungsten Carbide in Electrocatalytic Alcohol Oxidation. Chem. Mater. 2022, 34, 959–969. https://doi.org/10.1021/acs.chemmater.1c02535.
- 27.Kowal, A.; Port, S.N.; Nichols, R.J. Nickel Hydroxide Electrocatalysts for Alcohol Oxidation Reactions: An Evaluation by Infrared Spectroscopy and Electrochemical Methods. Catal. Today 1997, 38, 483–492. https://doi.org/10.1016/S0920-5861(97)00049-7.
- 28.Bender, M.T.; Lam, Y.C.; Hammes-Schiffer, S.; Choi, K.S. Unraveling Two Pathways for Electrochemical Alcohol and Aldehyde Oxidation on NiOOH. J. Am. Chem. Soc. 2021, 142, 21538–21547. https://doi.org/10.1021/jacs.0c10924.
- 29.Wang, T.J.; Huang, H.; Wu, X.R.; Yao, H.C.; Li, F.M.; Chen, P.; Jin, P.J.; Deng, Z.W.; Chen, Y. Selflate Synthesis of Defect-Rich NiO Nanotubes as Efficient Electrocatalysts for Methanol Oxidation Reaction. Nanoscale 2019, 11, 19783–19790. https://doi.org/10.1039/c9nr06304h.
- 30.Xu, Y.; Liu, M.; Wang, M.; Ren, T.; Ren, K.; Wang, Z.; Li, X.; Wang, L.; Wang, H. Methanol Electroreforming Coupled to Green Hydrogen Production over Bifunctional NiIr-Based Metal-Organic Framework Nanosheet Arrays. Appl. Catal. B Environ. 2022, 300, 120753. https://doi.org/10.1016/j.apcatb.2021.120753.
- 31.Li, J.; Ma, Y.; Yu, J.; Li, L.; Yang, H.; Gu, W.; Shi, J.; Wang, J.; Zhu, Y. Enhanced Methanol Electrooxidation and Supercapacitive Performance via Compositional Engineering of Colloidal Ni-Co Alloying Nanoparticles. ChemSusChem 2024, 18, e202401098. https://doi.org/10.1002/cssc.202401098.
- 32.Ma, Y.; Li, L.; Zhang, Y.; Jian, N.; Pan, H.; Deng, J.; Li, J. Nickel Foam Supported Mn-Doped NiFe-LDH Nanosheet Arrays as Efficient Bifunctional Electrocatalysts for Methanol Oxidation and Hydrogen Evolution. J. Colloid Interface Sci. 2024, 663, 971–980. https://doi.org/10.1016/j.jcis.2024.02.191.
- 33.Li, J.; Xing, C.; Zhang, Y.; Zhang, T.; Spadaro, M.C.; Wu, Q.; Yi, Y.; He, S.; Llorca, J.; Arbiol, J.; et al. Nickel Iron Diselenide for Highly Efficient and Selective Electrocatalytic Conversion of Methanol to Formate. Small 2021, 17, 2006623. https://doi.org/10.1002/smll.202006623.
- 34.Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. https://doi.org/10.1103/PhysRevLett.77.3865.
- 35.Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B-Condens. Matter Mater. Phys. 1996, 54, 11169–11186. https://doi.org/10.1103/PhysRevB.54.11169.
- 36.Meng, F.; Wu, Q.; Elouarzaki, K.; Luo, S.; Sun, Y.; Dai, C.; Xi, S.; Chen, Y.; Lin, X.; Fang, M.; et al. Essential Role of Lattice Oxygen in Methanol Electrochemical Refinery toward Formate. Sci. Adv. 2023, 9, eadh9487. https://doi.org/10.1126/sciadv.adh9487.
- 37.Zhou, H.; Ren, Y.; Li, Z.; Xu, M.; Wang, Y.; Ge, R.; Kong, X.; Zheng, L.; Duan, H. Electrocatalytic Upcycling of Polyethylene Terephthalate to Commodity Chemicals and H2 Fuel. Nat. Commun. 2021, 12, 4679. https://doi.org/10.1038/s41467-021-25048-x.
- 38.Friebel, D.; Louie, M.W.; Bajdich, M.; Sanwald, K.E.; Cai, Y.; Wise, A.M.; Cheng, M.J.; Sokaras, D.; Weng, T.C.; Alonso-Mori, R.; et al. Identification of Highly Active Fe Sites in (Ni,Fe)OOH for Electrocatalytic Water Splitting. J. Am. Chem. Soc. 2015, 137, 1305–1313. https://doi.org/10.1021/ja511559d.
- 39.Feng, X.; Liu, B.W.; Guo, K.X.; Fan, L.F.; Wang, G.X.; Ci, S.Q.; Wen, Z.H. Anodic Electrocatalysis of Glycerol Oxidation for Hybrid Alkali/Acid Electrolytic Hydrogen Generation. J. Electrochem. 2023, 29, 1–10. https://doi.org/10.13208/j.electrochem.2215005.
- 40.Chen, J.; Wang, K.; Liu, Z.; Sun, X.; Zhang, X.; Lei, F.; Wan, X.; Xie, J.; Tang, B. Sulfurization-Induced Lattice Disordering in High-Entropy Catalyst for Promoted Bifunctional Electro-Oxidation Behavior. Chem. Eng. J. 2024, 489, 151234. https://doi.org/10.1016/j.cej.2024.151234.
- 41.Li, J.; Yu, J.; Zhang, Y.; Li, C.; Ma, Y.; Ge, H.; Jian, N.; Li, L.; Zhang, C.Y.; Zhou, J.Y.; et al. Boosting Polysulfide Conversion on Fe-Doped Nickel Diselenide Toward Robust Lithium–Sulfur Batteries. Adv. Funct. Mater. 2025, 2501485. https://doi.org/10.1002/ADFM.202501485.
- 42.Li, H.H.; Xie, M.L.; Cui, C.H.; He, D.; Gong, M.; Jiang, J.; Zheng, Y.R.; Chen, G.; Lei, Y.; Yu, S.H. Surface Charge Polarization at the Interface: Enhancing the Oxygen Reduction via Precise Synthesis of Heterogeneous Ultrathin Pt/PtTe Nanowire. Chem. Mater. 2016, 28, 8890–8898. https://doi.org/10.1021/acs.chemmater.6b02769.
- 43.Trotochaud, L.; Young, S.L.; Ranney, J.K.; Boettcher, S.W. Nickel-Iron Oxyhydroxide Oxygen-Evolution Electrocatalysts: The Role of Intentional and Incidental Iron Incorporation. J. Am. Chem. Soc. 2014, 136, 6744–6753. https://doi.org/10.1021/ja502379c.
- 44.Phan, V.T.T.; Nguyen, Q.P.; Wang, B.; Burgess, I.J. Oxygen Vacancies Alter Methanol Oxidation Pathways on NiOOH. J. Am. Chem. Soc. 2024, 146, 4830–4841. https://doi.org/10.1021/jacs.3c13222.
- 45.Zhao, B.; Liu, J.; Xu, C.; Feng, R.; Sui, P.; Luo, J.X.; Wang, L.; Zhang, J.; Luo, J.L.; Fu, X.Z. Interfacial Engineering of Cu2Se/Co3Se4 Multivalent Hetero-Nanocrystals for Energy-Efficient Electrocatalytic Co-Generation of Value-Added Chemicals and Hydrogen. Appl. Catal. B Environ. 2021, 285, 119800. https://doi.org/10.1016/j.apcatb.2020.119800.
- 46.Zhan, C.; Bu, L.; Sun, H.; Huang, X.; Zhu, Z.; Yang, T.; Ma, H.; Li, L.; Wang, Y.; Geng, H.; et al. Medium/High-Entropy Amalgamated Core/Shell Nanoplate Achieves Efficient Formic Acid Catalysis for Direct Formic Acid Fuel Cell. Angew. Chem.-Int. Ed. 2023, 62, 1–8. https://doi.org/10.1002/anie.202213783.
- 47.Cao, C.; Ma, D.D.; Jia, J.; Xu, Q.; Wu, X.T.; Zhu, Q.L. Divergent Paths, Same Goal: A Pair-Electrosynthesis Tactic for Cost-Efficient and Exclusive Formate Production by Metal–Organic-Framework-Derived 2D Electrocatalysts. Adv. Mater. 2021, 33, 2008631. https://doi.org/10.1002/adma.202008631.
- 48.Deng, X.; Li, M.; Fan, Y.; Wang, L.; Fu, X.Z.; Luo, J.L. Constructing Multifunctional ‘Nanoplatelet-on-Nanoarray’ Electrocatalyst with Unprecedented Activity towards Novel Selective Organic Oxidation Reactions to Boost Hydrogen Production. Appl. Catal. B Environ. 2020, 278, 119339. https://doi.org/10.1016/j.apcatb.2020.119339.
- 49.Hao, J.; Liu, J.; Wu, D.; Chen, M.; Liang, Y.; Wang, Q.; Wang, L.; Fu, X.Z.; Luo, J.L. In Situ Facile Fabrication of Ni(OH)2 Nanosheet Arrays for Electrocatalytic Co-Production of Formate and Hydrogen from Methanol in Alkaline Solution. Appl. Catal. B Environ. 2021, 281, 119510. https://doi.org/10.1016/j.apcatb.2020.119510.
- 50.Wei, C.; Sun, S.; Mandler, D.; Wang, X.; Qiao, S.Z.; Xu, Z.J. Approaches for Measuring the Surface Areas of Metal Oxide Electrocatalysts for Determining Their Intrinsic Electrocatalytic Activity. Chem. Soc. Rev. 2019, 48, 2518–2534. https://doi.org/10.1039/c8cs00848e.
- 51.Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2001.
- 52.Zhao, B.; Liu, J.; Wang, X.; Xu, C.; Sui, P.; Feng, R.; Wang, L.; Zhang, J.; Luo, J.L.; Fu, X.Z. CO2-Emission-Free Electrocatalytic CH3OH Selective Upgrading with High Productivity at Large Current Densities for Energy Saved Hydrogen Co-Generation. Nano Energy 2021, 80, 105530. https://doi.org/10.1016/j.nanoen.2020.105530.
- 53.Sun, S.N.; Dong, L.Z.; Li, J.R.; Shi, J.W.; Liu, J.; Wang, Y.R.; Huang, Q.; Lan, Y.Q. Redox-Active Crystalline Coordination Catalyst for Hybrid Electrocatalytic Methanol Oxidation and CO2 Reduction. Angew. Chem.-Int. Ed. 2022, 61, e202207282. https://doi.org/10.1002/anie.202207282.
- 54.Ganguly, S.; Paul, S.; Khurana, D.; Khan, T.S.; Giri, P.K.; Loha, C.; Ghosh, S. Ternary Ni-Co-Se Nanostructure for Electrocatalytic Oxidative Value Addition of Biomass Platform Chemicals. ACS Appl. Energy Mater. 2023, 6, 5331–5341. https://doi.org/10.1021/acsaem.3c00313.
- 55.Liu, B.; Wang, X.; Wang, S.; Peng, H.Q.; Xiao, T.; Liu, G.; Bai, S.; Zhao, Y.; Zhang, W.; Song, Y.F. Hydroxyl Vacancies Triggered High Methanol Oxidation Activity of Monolayered Layered Double Hydroxides for Energy-Saving Hydrogen Production. Mater. Today Energy 2022, 28, 101082. https://doi.org/10.1016/j.mtener.2022.101082.
- 56.Schimpf, A.M.; Knowles, K.E.; Carroll, G.M.; Gamelin, D.R. Electronic Doping and Redox-Potential Tuning in Colloidal Semiconductor Nanocrystals. Acc. Chem. Res. 2015, 48, 1929–1937. https://doi.org/10.1021/acs.accounts.5b00181.
- 57.Si, F.; Liu, J.; Zhang, Y.; Zhao, B.; Liang, Y.; Wu, X.; Kang, X.; Yang, X.; Zhang, J.; Fu, X.Z.; et al. Surface Spin Enhanced High Stable NiCo2S4 for Energy-Saving Production of H2 from Water/Methanol Coelectrolysis at High Current Density. Small 2023, 19, 2205257. https://doi.org/10.1002/smll.202205257.
- 58.Li, J.; Li, L.; Ma, X.; Han, X.; Xing, C.; Qi, X.; He, R.; Arbiol, J.; Pan, H.; Zhao, J.; et al. Selective Ethylene Glycol Oxidation to Formate on Nickel Selenide with Simultaneous Evolution of Hydrogen. Adv. Sci. 2023, 10, 2300841. https://doi.org/10.1002/advs.202300841.
- 59.Abdullah, M.I.; Hameed, A.; Zhang, N.; Islam, M.H.; Ma, M.; Pollet, B.G. Ultrasonically Surface-Activated Nickel Foam as a Highly Efficient Monolith Electrode for the Catalytic Oxidation of Methanol to Formate. ACS Appl. Mater. Interfaces 2021, 13, 30603–30613. https://doi.org/10.1021/acsami.1c06258.
- 60.Mondal, B.; Karjule, N.; Singh, C.; Shimoni, R.; Volokh, M.; Hod, I.; Shalom, M. Unraveling the Mechanisms of Electrocatalytic Oxygenation and Dehydrogenation of Organic Molecules to Value-Added Chemicals Over a Ni–Fe Oxide Catalyst. Adv. Energy Mater. 2021, 11, 2101858. https://doi.org/10.1002/AENM.202101858.
- 61.Cui, X.; Xiao, P.; Wang, J.; Zhou, M.; Guo, W.; Yang, Y.; He, Y.; Wang, Z.; Yang, Y.; Zhang, Y.; et al. Highly Branched Metal Alloy Networks with Superior Activities for the Methanol Oxidation Reaction. Angew. Chem.- Int. Ed. 2017, 56, 4488–4493. https://doi.org/10.1002/anie.201701149.
- 62.Li, J.; Tian, X.; Wang, X.; Zhang, T.; Spadaro, M.C.; Arbiol, J.; Li, L.; Zuo, Y.; Cabot, A. Electrochemical Conversion of Alcohols into Acidic Commodities on Nickel Sulfide Nanoparticles. Inorg. Chem. 2022, 61, 13433–13441. https://doi.org/10.1021/acs.inorgchem.2c01695.
- 63.Candelaria, S.L.; Bedford, N.M.; Woehl, T.J.; Rentz, N.S.; Showalter, A.R.; Pylypenko, S.; Bunker, B.A.; Lee, S.; Reinhart, B.; Ren, Y.; et al. Multi-Component Fe-Ni Hydroxide Nanocatalyst for Oxygen Evolution and Methanol Oxidation Reactions under Alkaline Conditions. ACS Catal. 2017, 7, 365–379. https://doi.org/10.1021/acscatal.6b02552.
- 64.Zhao, B.; Xu, C.; Shakouri, M.; Feng, R.; Zhang, Y.; Liu, J.; Wang, L.; Zhang, J.; Luo, J.-L. L.; Fu, X.-Z. Z. Anode-Cathode Interchangeable Strategy for in Situ Reviving Electrocatalysts’ Critical Active Sites for Highly Stable Methanol Upgrading and Hydrogen Evolution Reactions. Appl. Catal. B Environ. 2022, 305, 121082. https://doi.org/10.1016/j.apcatb.2022.121082.
- 65.Li, J. Nickel-Organic Frameworks as Highly Efficient Catalyst for Electrochemical Conversion of CH3OH into Formic Acid. Electrochem. commun. 2023, 146, 107416. https://doi.org/10.1016/j.elecom.2022.107416.
- 66.Arshad, F.; ul Haq, T.; Khan, A.; Haik, Y.; Hussain, I.; Sher, F. Multifunctional Porous NiCo Bimetallic Foams toward Water Splitting and Methanol Oxidation-Assisted Hydrogen Production. Energy Convers. Manag. 2022, 254, 115262. https://doi.org/10.1016/j.enconman.2022.115262.
How to Cite
Jian, N.; Ge, H.; Ma, Y.; Zhang, Y.; Li, L.; Liu, J.; Yu, J.; Li, C.; Li, J. Improved Methanol-to-Formate Electrocatalytic Reaction by Engineering of Nickel Hydroxide and Iron Oxyhydroxide Heterostructures. Science for Energy and Environment 2025, 2 (1), 3. https://doi.org/10.53941/see.2025.100003.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


