Coral reefs biodiversity and productivity are currently in decline due to the impacts of human activities, especially those associated with chemical pollutants, including metals. In this context, iron (Fe) contamination of coastal waters associated with land runoff and disasters associated with mining activities has drawn attention around the globe, especially in the Southern Atlantic coast. Fe is an essential metal involved in photosynthesis, respiration, and oxidative metabolism, which can thus influence parameters associated with photosynthesis and the activity of ATPases. Therefore, we evaluated the acute and chronic effects of Fe on the maximum quantum yield of photosystem II and carbonic anhydrase and Ca2+-ATPase activities in three corals species: Mussismilia harttii, Siderastrea sp., and Millepora alcicornis. Corals were maintained in control condition (no Fe addition in seawater) and acutely (4 days—laboratory conditions) or chronically (up to 28 days—mesocosm conditions) exposed to different increments of Fe (0.1, 0.3, and 0.9 mg L−1) in seawater. The tested concentrations were selected based on the range of total and dissolved Fe concentrations observed in seawater in reef environments of the South Atlantic Ocean after the collapse of the Fundão mine dam occurred in Mariana (state of Minas Gerais, southeastern Brazil) in 2015. In the acute and chronic experiments, three and four replicates were performed for each experimental condition, respectively. In the acute exposure, all biological parameters were measured after 4 days of exposure. In the chronic exposure, the maximum quantum yield of photosystem II was measured at 5, 10, 17, and 24 days of exposure while enzyme activities were analyzed at 14 and 28 days of exposure. Results indicated that the maximum quantum yield of photosystem II was decreased by 20.5% (p < 0.05) in Mi. alcicornis exposed for 17 days to 0.1 mg L−1 Fe, when compared to the control condition at the same experimental time. Along the experimental time, it was decreased (p < 0.05) by 19.8% and 20.9% in Mu. harttii exposed for 24 days to 0.3 and 0.9 mg L−1 Fe, respectively. In Mu. harttii, carbonic anhydrase activity was reduced by 31.7% after acute exposure of corals to 0.3 mg L−1 Fe and increased by 102.4% when they were exposed to 0.9 mg L−1 Fe. Also, carbonic anhydrase activity was reduced (p < 0.05) by 62.1% and 54.5% in Mi. alcicornis exposed for 14 days to 0.3 and 0.9 mg L−1 Fe, respectively. After 28 days of Fe exposure, no significant change is CA activity was observed in the three species of corals. Furthermore, Ca2+-ATPase activity of the three coral species was not altered by the Fe increments in seawater, regardless of the exposure time. Overall, our findings indicates that exposure to increments of Fe in seawater influenced the health- (maximum quantum yield of photosystem II) and growth-related (carbonic anhydrase activity) biomarkers evaluated. The observed effects were specific to the three coral species tested and highlight the need to test the impacts of the seawater contamination with Fe over longer exposure periods than those tested in the present study.
- Open Access
- Article
Responses of Health- and Growth-Related Biomarkers in Corals Exposed to Iron
- Juliana da Silva Fonseca 1,
- Letícia May Fukushima 1,
- João Vitor Langorte Bueno 2,
- Thales Jean Vidal 3,
- Kely Paula Salvi 4,
- Carlos Henrique Figueiredo Lacerda 4,
- Patrícia Gomes Costa 1,
- Miguel Mies 4,5,
- Adalto Bianchini 4,6,*
Author Information
Received: 12 Oct 2025 | Revised: 09 Dec 2025 | Accepted: 15 Dec 2025 | Published: 24 Dec 2025
Abstract
Graphical Abstract
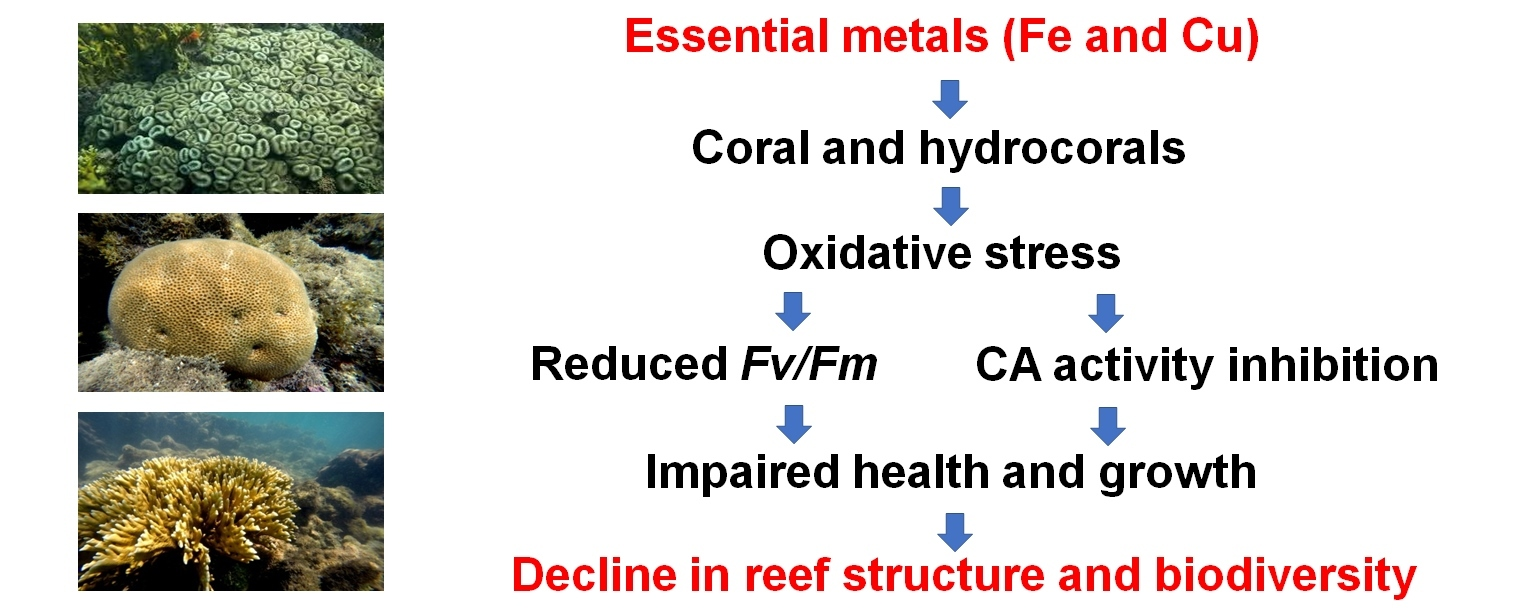
Keywords
calcification | coral reefs | environmental contamination | metal | Fundão dam collapse | Fv/Fm
References
- 1.
Hatcher, B.G. Coral reef primary productivity. A hierarchy of pattern and process. Trends Ecol. Evol. 1990, 5, 149‒155. https://doi.org/10.1016/0169-5347(90)90221-X.
- 2.
Davis, K.L.; Colefax, A.P.; Tucker, J.P.; et al. Global coral reef ecosystems exhibit declining calcification and increasing primary productivity. Commun. Earth Environ. 2021, 2, 105. https://doi.org/10.1038/s43247-021-00168-w.
- 3.
Fisher, R.; O’Leary, R.A.; Low-Choy, S.; et al. Species richness on coral reefs and the pursuit of convergent global estimates. Curr. Biol. 2015, 25, 500‒505. https://doi.org/10.1016/j.cub.2014.12.022.
- 4.
Costanza, R.; De Groot, R.; Sutton, P.; et al. Changes in the global value of ecosystem services. Global Environ. Chang. 2014, 26, 152‒158. https://doi.org/10.1016/j.gloenvcha.2014.04.002.
- 5.
Giglio, V.J.; Aued, A.W.; Cordeiro, C.A.; Eggertsen, L.; et al. A global systematic literature review of ecosystem services in reef environments. Environ. Manag. 2024, 73, 634‒645. https://doi.org/10.1007/s00267-023-01912-y.
- 6.
Leão, Z.M.A.N.; Kikuchi, R.K.P.; Ferreira, B.P.; et al. Brazilian coral reefs in a period of global change: A synthesis. Braz. J. Oceanogr. 2016, 64, 97‒116. https://doi.org/10.1590/S1679-875920160916064sp2.
- 7.
Pinheiro, H.T.; Rocha, L.A.; Macieira, R.M.; et al. South-western Atlantic reef fishes: Zoogeographical patterns and ecological drivers reveal a secondary biodiversity centre in the Atlantic Ocean. Divers. Distrib. 2018, 24, 951–965. http://dx.doi.org/10.1111/ddi.12729.
- 8.
Castro, C.B.; Pires, D.O. Brazilian coral reefs: What we already know and what is still missing. Bull. Mar. Sci. 2001, 69, 357–371.
- 9.
Pereira, P.H.; Lima, G.V.; Araujo, J.C.; et al. Mesophotic reefs of the largest Brazilian coastal protected area: Mapping, characterization and biodiversity. Diversity 2022, 14, 760. https://doi.org/10.3390/d14090760.
- 10.
Coni, E.O.; Ferreira, C.M.; Moura, R.L.; et al. An evaluation of the use of branching fire-corals (Millepora spp.) as refuge by reef fish in the Abrolhos Bank, eastern Brazil. Environ. Biol. Fishes 2013, 96, 45‒55. https://doi.org/10.1007/s10641-012-0021-6.
- 11.
Luza, A.L.; Quimbayo, J.P.; Ferreira, C.E.; et al. Low functional vulnerability of fish assemblages to coral loss in Southwestern Atlantic marginal reefs. Sci. Rep. 2022, 12, 17164. https://doi.org/10.1038/s41598-022-20919-9.
- 12.
Allemand, D.; Ferrier-Pagès, C.; Furla, P.; et al. Biomineralisation in reef-building corals: From molecular mechanisms to environmental control. C. R. Palevol. 2004, 3, 453–467. https://doi.org/10.1016/j.crpv.2004.07.011.
- 13.
Allemand, D.; Tambutté, E.; Zoccola, D.; et al. Coral calcification, cells to reefs. In Coral Reefs: An Ecosystem in Transition; Dubinsky, Z., Stambler, N., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 119‒150. https://doi.org/10.1007/978-94-007-0114-4.
- 14.
Bertucci, A.; Moya, A.; Tambutté, S.; et al. Carbonic anhydrases in anthozoan corals e a review. Bioorg. Med. Chem. 2013, 21, 1437‒1450. https://doi.org/10.1016/j.bmc.2012.10.024.
- 15.
Rollion-Bard, C.; Blamart, D. Possible controls on Li, Na, and Mg incorporation into aragonite coral skeletons. Chem. Geol. 2015, 396, 98–111. https://doi.org/10.1016/j.chemgeo.2014.12.011.
- 16.
Al-Horani, F.A.; Al-Moghrabi, S.M.; De Beer, D. The mechanism of calcification and its relation to photosynthesis and respiration in the scleractinian coral Galaxea fascicularis. Mar. Biol. 2003, 142, 419‒426. http://dx.doi.org/10.1007/s00227-002-0981-8.
- 17.
Cohen, A.L.; McConnaughey, T.A. A geochemical perspective on coral mineralization. Rev. Mineral. Geochem. 2003, 54, 151‒187. http://dx.doi.org/10.2113/0540151.
- 18.
LaJeunesse, T.C.; Parkinson, J.E.; Gabrielson, P.W.; Jeong, H.J.; Reimer, J.D.; Voolstra, C.R.; Santos, S.R. Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr. Biol. 2018, 28, 2570‒2580. https://doi.org/10.1016/j.cub.2018.07.008.
- 19.
Colombo-Pallotta, M.F.; Rodríguez-Román, A.; Iglesias-Prieto, R. Calcification in bleached and unbleached Montastraea faveolata: Evaluating the role of oxygen and glycerol. Coral Reefs 2010, 29, 899‒907. https://doi.org/10.1007/s00338-010-0638-x.
- 20.
Davy, S.K.; Allemand, D.; Weis, V.M. Cell biology of cnidarian-dinoflagellate symbiosis. Microbiol. Mol. Biol. 2012, 76, 229‒261. https://doi.org/10.1128/mmbr.05014-11.
- 21.
Muscatine, L.; Porter, J.W. Reef corals: Mutualistic symbioses adapted to nutrient-poor environments. Bioscience 1977, 27, 454–460. https://doi.org/10.2307/1297526.
- 22.
Stanley, G.D. Photosymbiosis and the evolution of modern coral reefs. Science 2006, 312, 857–858. https://doi.org/10.1126/science.1123701.
- 23.
Fonseca, J.S.; Marangoni, L.F.; Marques, J.A.; et al. Effects of increasing temperature alone and combined with copper exposure on biochemical and physiological parameters in the zooxanthellate scleractinian coral Mussismilia harttii. Aquat. Toxicol. 2017, 190, 121‒132. https://doi.org/10.1016/j.aquatox.2017.07.002.
- 24.
Jones, R.J.; Kildea, T.; Hoegh-Guldberg, O. PAM chlorophyll fluorometry: A new in situ technique for stress assessment in scleractinian corals, used to examine the effects of cyanide from cyanide fishing. Mar. Pollut. Bull. 1999, 38, 864‒874. https://doi.org/10.1016/S0025-326X(98)90160-6.
- 25.
Silverstein, R.N.; Cunning, R.; Baker, A.C. Change in algal symbiont communities after bleaching, not prior heat exposure, increases heat tolerance of reef corals. Glob. Chang. Biol. 2014, 21, 236‒249. https://doi.org/10.1111/gcb.12706.
- 26.
van Dam, J.W.; Negri, A.P.; Uthicke, S.; Mueller, J.F. Chemical pollution on coral reefs: Exposure and ecological effects. In Ecological Impacts of Toxic Chemicals; Sanchez-Bayo, F., van den Brink, P.J., Mann, R.M., Eds.; Bentham Science Publishers: Amsterdam, The Netherlands, 2011; Chapter 9, pp. 187‒211. https://doi.org/10.2174/978160805121210187.
- 27.
McLean, M.; Cuetos-Bueno, J.; Nedlic, O.; et al. Local stressors, resilience, and shifting baselines on coral reefs. PLoS ONE 2016, 11, 0166319. https://doi.org/10.1371/journal.pone.0166319.
- 28.
Harborne, A.R.; Rogers, A.; Bozec, Y.M.; et al. Multiple stressors and the functioning of coral reefs. Ann. Rev. Mar. Sci. 2017, 9, 445‒468. https://doi.org/10.1146/annurev-marine-010816-060551.
- 29.
Ellis, J.I.; Jamil, T.; Anlauf, H.; et al. Multiple stressor effects on coral reef ecosystems. Glob. Chang. Biol. 2019, 25, 4131‒4146. https://doi.org/10.1111/gcb.14819.
- 30.
Hughes, T.P.; Anderson, K.D.; Connolly, S.R.; et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 2018, 359, 80‒83. https://doi.org/10.1126/science.aan8048.
- 31.
Dietzel, A.; Bode, M.; Connolly, S.R.; et al. Long-term shifts in the colony size structure of coral populations along the Great Barrier Reef. Proc. R. Soc. B 2020, 287, 20201432. https://doi.org/10.1098/rspb.2020.1432.
- 32.
Tebbett, S.B.; Connolly, S.R.; Bellwood, D.R. Benthic composition changes on coral reefs at global scales. Nat. Ecol. Evol. 2023, 7, 71‒81. https://doi.org/10.1038/s41559-022-01937-2.
- 33.
Prouty, N.G.; Cohen, A.; Yates, K.K.; et al. Vulnerability of coral reefs to bioerosion from land-based sources of pollution. J. Geophys. Res. Oceans 2017, 122, 9319‒9331. https://doi.org/10.1002/2017jc013264.
- 34.
Silbiger, N.J.; Nelson, C.E.; Remple, K.; et al. Nutrient pollution disrupts key ecosystem functions on coral reefs. Proc. R. Soc. B 2018, 285, 20172718. https://doi.org/10.1098/rspb.2017.2718.
- 35.
Dubinsky, Z.; Stambler, N. Marine pollution and coral reefs. Glob. Chang. Biol. 1996, 2, 511‒526. https://doi.org/10.1111/j.1365-2486.1996.tb00064.x.
- 36.
Jones, R.J. Zooxanthellae loss as a bioassay for assessing stress in corals. Mar. Ecol. Prog. Ser. 1997, 149, 163‒171. https://doi.org/10.3354/meps149163.
- 37.
Verma, R.; Dwivedi, P. Heavy metal water pollution—A case study. Rec. Res. Sci. Technol. 2013, 5, 98‒99.
- 38.
Francini-Filho, R.B.; Cordeiro, M.C.; Omachi, C.Y.; et al. Remote sensing, isotopic composition and metagenomics analyses revealed Doce River ore plume reached the southern Abrolhos Bank Reefs. Sci. Total Environ. 2019, 697, 134038. https://doi.org/10.1016/j.scitotenv.2019.134038.
- 39.
Magris, R.A.; Marta-Almeida, M.; Monteiro, J.A.F.; et al. A modelling approach to assess the impact of land mining on marine biodiversity: Assessment in coastal catchments experiencing catastrophic events (SW Brazil). Sci. Total Environ. 2019, 659, 828–840. https://doi.org/10.1016/j.scitotenv.2018.12.238.
- 40.
Coimbra, K.T.O.; Alcântara, E.; de Souza Filho, C.R. Possible contamination of the Abrolhos reefs by Fundao dam tailings, Brazil—New constraints based on satellite data. Sci. Total Environ. 2020, 733, 138101. https://doi.org/10.1016/j.scitotenv.2020.138101.
- 41.
Evangelista, H.; de Paula, R.L.M.; Magalhães, N.; et al. Intake of trace contaminants by corals in Abrolhos reef bank (western South Atlantic) during two decades of coastal impacts. Cont. Shelf Res. 2023, 255, 104946. https://doi.org/10.1016/j.csr.2023.104946.
- 42.
Leão, Z.M.A.N.; Kikuchi, R.K.P. The Abrolhos reefs of Brazil. In Coastal Marine Ecosystems of Latin America. Ecological Studies; Seeliger, U., Kjerfve, B., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2001; Volume 144, pp. 83–96. https://doi.org/10.1007/978-3-662-04482-7_7.
- 43.
Dutra, G.F.; Santos, L.P.; Coutinho, B.H.; et al. Marine biodiversity hotspots in the Abrolhos Region and Vitória-Trindade Seamount Chain, Brazil, with implications for conservation. Ocean Coast. Res. 2025, 73, e25019. https://doi.org/10.1590/2675-2824073.24055.
- 44.
Dal Pizzol, J.L.; Marques, J.A.; Fonseca, J.S.; et al. Metal accumulation induces oxidative stress and alters carbonic anhydrase activity in corals and symbionts from the largest reef complex in the South Atlantic ocean. Chemosphere 2022, 290, 133216. https://doi.org/10.1016/j.chemosphere.2021.133216.
- 45.
Cardoso, G.O.; Falsarella, L.N.; Chiroque-Solano, P.M.; et al. Coral growth bands recorded trace elements associated with the Fundão dam collapse. Sci. Total Environ. 2021, 807, 150880. https://doi.org/10.1016/j.scitotenv.2021.150880.
- 46.
Costa, P.G.; Marube, L.C.; Artifon, V.; et al. Temporal and spatial variations in metals and arsenic contamination in water, sediment and biota of freshwater, marine and coastal environments after the Fundão dam failure. Sci. Total Environ. 2022, 806, 151340. https://doi.org/10.1016/j.scitotenv.2021.151340.
- 47.
Romero, J.; Tonetti Botana, M.; Elias, A.; et al. Effect of iron speciation on growth and heat resistance of Symbiodiniaceae. Ocean Coast. Res. 2022, 70, 22016. https://doi.org/10.1590/2675-2824070.21103jmdr.
- 48.
Dellisanti, W.; Zhang, Q.; Ferrier-Pagès, C.; et al. Contrasting effects of increasing dissolved iron on photosynthesis and O2 availability in the gastric cavity of two Mediterranean corals. PeerJ. 2024, 12, e17259. https://doi.org/10.7717/peerj.17259.
- 49.
Tu, T.-H.; Hsieh, H.-Y.; Meng, P.-J.; et al. Physiological responses of scleractinian coral to trace metal enrichment and thermal stress. Mar. Environ. Res. 2025, 207, 107085. https://doi.org/10.1016/j.marenvres.2025.107085.
- 50.
Grant, A.J.; Graham, K.; Frankland, S.; et al. Effect of copper on algal-host interactions in the symbiotic coral Plesiastrea versipora. Plant Physiol. Biochem. 2003, 41, 383‒390. https://doi.org/10.1016/S0981-9428(03)00034-2.
- 51.
Bielmyer, G.K.; Grosell, M.; Bhagooli, R.; et al. Differential effects of copper on three species of scleractinian corals and their algal symbionts (Symbiodinium spp.). Aquat. Toxicol. 2010, 97, 125‒133. https://doi.org/10.1016/j.aquatox.2009.12.021.
- 52.
Yost, D.M.; Jones, R.J.; Mitchelmore, C.L. Alterations in dimethylsulfoniopropionate (DMSP) levels in the coral Montastraea franksi in response to copper exposure. Aquat. Toxicol. 2010, 98, 367‒373. https://doi.org/10.1016/j.aquatox.2010.03.005.
- 53.
Schwarz, J.A.; Mitchelmore, C.L.; Jones, R.; et al. Exposure to copper induces oxidative and stress responses and DNA damage in the coral Montastraea franksi. Comp. Biochem. Physiol. C 2013, 157, 272‒279. https://doi.org/10.1016/j.cbpc.2012.12.003.
- 54.
Fonseca, J.S.; Marangoni, L.F.B.; Marques, J.A.; Bianchini, A. Carbonic anhydrase as a potential biomarker for acute exposure to copper in corals. Chemosphere 2019, 227, 598‒605. https://doi.org/10.1016/j.chemosphere.2019.04.089.
- 55.
Nystrom, M.; Nordemar, I.; Tedengren, M. Simultaneous and sequential stress from increased temperature and copper on the metabolism of the hermatypic coral Porites cylindrical. Mar. Biol. 2001, 138, 1225‒1231. https://doi.org/10.1007/s002270100549.
- 56.
Negri, A.P.; Hoogenboom, M.O. Water contamination reduces the tolerance of coral larvae to thermal stress. PLoS ONE 2011, 6, e19703. https://doi.org/10.1371/journal.pone.0019703.
- 57.
Nikinmaa, M. Climate change and ocean acidification interactions with aquatic toxicology. Aquat. Toxicol. 2013, 126, 365‒372. https://doi.org/10.1016/j.aquatox.2012.09.006.
- 58.
Fonseca, J.S.; Marangoni, L.F.B.; Marques, J.A.; et al. Energy metabolism enzymes inhibition by the combined effects of increasing temperature and copper exposure in the coral Mussismilia harttii. Chemosphere 2019, 236, 124420. https://doi.org/10.1016/j.chemosphere.2019.124420.
- 59.
Winterbourn, C.C. Toxicity of iron and hydrogen peroxide: The Fenton reaction. Toxicol. Lett. 1995, 82/83, 969–974. https://doi.org/10.1016/0378-4274(95)03532-X.
- 60.
Liu, R.; Liu, W.; Doctrow, S.R.; et al. Iron toxicity in organotypic cultures of hippocampal slices: role of reactive oxygen species. Neurobiol. Aging 2003, 24, 977–983. https://doi.org/10.1046/j.1471-4159.2003.01708.x.
- 61.
Vijayavel, K.; Downs, C.A.; Ostrander, G.K.; et al. Oxidative DNA damage induced by iron chloride in the larvae of the lace coral Pocillopora damicornis. Comp. Biochem. Physiol. C 2012, 155, 275‒280. https://doi.org/10.1016/j.cbpc.2011.09.007.
- 62.
Lesser, M.P. Oxidative stress in marine environments: Biochemistry and physiological ecology. Annu. Rev. Physiol. 2006, 68, 253‒278. https://doi.org/10.1146/annurev.physiol.68.040104.110001.
- 63.
Vajreswari, A.; Rao, P.S.; Kaplay, S.S.; et al. Erythrocyte membrane in rats fed high erucic acid-containing mustard oil: Osmotic fragility, lipid composition, and (Na+, K+)- and (Ca2+, Mg2+)-ATPases. Biochem. Med. 1983, 29, 74–84. https://doi.org/10.1016/0006-2944(83)90056-x.
- 64.
Kaya, E.D.; Soyüt, H.; Beydemir, S. Carbonic anhydrase activity from the gilthead seabream (Sparus aurata) liver: The toxicological effects of heavy metals. Environ. Toxicol. Pharmacol. 2013, 36, 514–521. https://doi.org/10.1016/j.etap.2013.05.019.
- 65.
Kaya, E.D.; Soyüt, H.; Beydemir, S. The toxicological impacts of some heavy metals on carbonic anhydrase from gilthead sea bream (Sparus aurata) gills. Environ. Toxicol. Pharmacol. 2015, 39, 825–832. https://doi.org/10.1016/j.etap.2015.01.021.
- 66.
Lionetto, M.G.; Caricato, R.; Erroi, E.; et al. Potential application of carbonic anhydrase activity in bioassay and biomarker studies. Chem. Ecol. 2006, 22, S119–S125. https://doi.org/10.1080/02757540600670661.
- 67.
Marangoni, L.F.B.; Marques, J.A.; Duarte, G.A.S.; et al. Copper effects on biomarkers associated with photosynthesis, oxidative status and calcification in the Brazilian coral Mussismilia harttii (Scleractinia, Mussidae). Mar. Environ. Res. 2017, 130, 248‒257. https://doi.org/10.1016/j.marenvres.2017.08.002.
- 68.
Ross, C.L.; DeCarlo, T.M.; McCulloch, M.T. Environmental and physiochemical controls on coral calcification along a latitudinal temperature gradient in Western Australia. Glob. Chang. Biol. 2019, 25, 431‒447. https://doi.org/10.1111/gcb.14488.
- 69.
Venn, A.A.; Tambutté, E.; Zoccola, D.; et al. Coral calcification at the cellular scale: Insight through the ‘window’ of the growing edge. In Frontiers in Invertebrate Physiology: A Collection of Reviews; Saleuddin, S., Leys, S.P., Roer, R.D., et al., Eds.; Apple Academic Press: New York, NY, USA, 2024; pp. 343‒397. https://doi.org/10.1201/9781003403319-7.
- 70.
De’ath, G.; Lough, J.M.; Fabricius, K.E. Declining coral calcification on the great barrier reef. Science 2009, 323, 116‒119. https://doi.org/10.1126/science.1165283.
- 71.
Weis, V.M.; Allemand, D. What determines coral health? Science 2009, 324, 1153‒1155. https://doi.org/10.1126/science.1172540.
- 72.
Grottoli, A.G.; Toonen, R.J.; van Woesik, R.; et al. Increasing comparability among coral bleaching experiments. Ecol. Appl. 2021, 31, e02262. https://doi.org/10.1002/eap.2262.
- 73.
Armstrong, F.A.J. The iron content of sea water. J. Mar. Biol. Assoc. U.K. 1957, 36, 509–517. https://doi.org/10.1017/S0025315400025807.
- 74.
Bianchini, A.; Silva, C.C.; Lauer, M.M.; et al. Avaliação do Impacto da Lama/Pluma Samarco sobre os Ambientes Costeiros e Marinhos (ES e BA) com Ênfase nas Unidades de Conservação: 1a Expedição do Navio de Pesquisa Soloncy Moura do CEPSUL/ICMBio. 2016. Available online: https://www.gov.br/icmbio/pt-br/centrais-de-conteudo/publicacoes/documentos/documentos-rio-doce-espirito-santo/DCOM_relatorio_revisado_atualizado_29_04_2016_AB.pdf (accessed on 19 September 2025).
- 75.
Sá, F.; Longhini, C.M.; Costa, E.S.; et al. Time-sequence development of metal (loid)s following the 2015 dam failure in the Doce river estuary, Brazil. Sci. Total Environ. 2021, 769, 144532. https://doi.org/10.1016/J.SCITOTENV.2020.144532.
- 76.
Longhini, C.M.; Rodrigues, S.K.; Costa, E.S.; et al. Environmental quality assessment in a marine coastal area impacted by mining tailing using a geochemical multi-index and physical approach. Sci. Total Environ., 2022, 803, 149883. https://doi. org/10.1016/J.SCITOTENV.2021.149883.
- 77.
PMBA. Programa de Monitoramento da Biodiversidade Aquática da Área Ambiental I—Porção Capixaba do Rio Doce e Região Marinha e Costeira Adjacente, RT-51/SET 25, RSE2025 PMBA/Fest-UFES; Fundação Espírito-santense de Tecnologia: Vitória, ES, Brazil, 2025.
- 78.
Duarte, G.; Calderon, E.N.; Pereira, C.M.; et al. A novel marine mesocosm facility to study global warming, water quality, and ocean acidification. Ecol. Evol. 2015, 5, 4555‒4566. https://doi.org/10.1002/ece3.1670.
- 79.
Bianchini, A.; Fukushima, L.M.; Grillo, A.C.; et al. Marine mesocosm system: A reliable tool for testing bioaccumulation and effects of iron in reef organisms. MethodsX 2024, 13, 102949. https://doi.org/10.1016/j.mex.2024.102949.
- 80.
Macedo, R.S.; Lombardi, A.T.; Omachi, C.Y.; et al. Effects of the herbicide bentazon on growth and photosystem II maximum quantum yield of the marine diatom Skeletonema costatum. Toxicol. Vitr. 2008, 22, 716‒722. https://doi.org/10.1016/j.tiv.2007.11.012.
- 81.
Downs, C.A.; Fouth, J.E.; Robinson, C.E.; et al. Cellular diagnostics and coral health: Declining coral health in the Florida Keys. Mar. Pollut. Bull. 2005, 51, 558‒569. https://doi.org/10.1016/j.marpolbul.2005.04.017.
- 82.
Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248‒254. https://doi.org/10.1016/0003-2697(76)90527-3.
- 83.
Henry, R.P. Techniques for measuring carbonic anhydrase activity in vitro: The electrometric delta pH and pH stat methods. In The Carbonic Anhydrases: Cellular Physiology and Molecular Genetics; Dodgson, S.J., Tashian, R.E., Gros, G., et al., Eds.; Springer: New York, NY, USA, 1991; pp. 119–125. https://doi.org/10.1007/978-1-4899-0750-9.
- 84.
Harland, A.D.; Brown, B.E. Metal tolerance in the scleractinian coral Porites lutea. Mar. Pollut. Bull. 1989, 20, 353–357. https://doi.org/10.1016/0025-326X(89)90159-8.
- 85.
Rädecker, N.; Pogoreutz, C.; Ziegler, M.; et al. Assessing the effects of iron enrichment across holobiont compartments reveals reduced microbial nitrogen fixation in the Red Sea coral Pocillopora verrucosa. Ecol. Evol. 2017, 7, 6614‒6621. https://doi.org/10.1002/ece3.3293.
- 86.
Howeler, R.H. Iron induced ringing disease of rice in relation to physicochemical changes in a flooded soil. Soil Sci. Soc. Am. J. 1973, 37, 898‒903. https://doi.org/10.2136/sssaj1973.03615995003700060030x.
- 87.
Sahrawat, K. Iron Toxicity in Wetland Rice and the Role of Other Nutrients. J. Plant Nutr. 2005, 27, 1471‒1504. https://doi.org/10.1081/PLN-200025869.
- 88.
Arunachalam, R.; Paulkumar, K.; Ranjitsingh, A.J.A.; et al. Environmental assessment due to air pollution near iron smelting industry. J. Environ. Sci. Technol. 2009, 2, 179‒186. https://doi.org/10.3923/jest.2009.179.186.
- 89.
Rout, G.R.; Sahoo, S. Role of iron in plant growth and metabolism. Rev. Agric. Sci. 2015, 3, 1‒24. https://doi.org/10.7831/ras.3.1.
- 90.
Papanikolaou, G.; Pantopoulos, K. Iron metabolism and toxicity. Toxicol Appl Pharmacol. 2005, 202, 199‒211. https://doi.org/10.1016/j.taap.2004.06.021.
- 91.
Aisen, P.; Enns, C.; Wessling-Resnick, M. Chemistry and biology of eukaryotic iron metabolism. Int. J. Biochem. Cell Biol. 2001, 33, 940–959. https://doi.org/10.1016/s1357-2725(01)00063-2.
- 92.
Ferrier-Pagès, C.; Schoelzke, V.; Jaubert, J.; et al. Response of a scleractinian coral, Stylophora pistillata, to iron and nitrate enrichment. J. Exp. Mar. Biol. Ecol. 2001, 259, 249–261. https://doi.org/10.1016/S0022-0981(01)00241-6.
- 93.
Rodriguez, I.; Lin, S.; Ho, J.; et al. Effects of trace metal concentrations on the growth of the coral endosymbiont Symbiodinium. Front. Microbiol. 2016, 7, 82. https://doi.org/10.3389/fmicb.2016.00082.
- 94.
Reich, H.G.; Tu, W.C.; Rodriguez, I.B.; et al. Iron availability modulates the response of endosymbiotic dinoflagellates to heat stress. J. Phycol. 2021, 57, 3‒13. https://doi.org/10.1111/jpy.13078.
- 95.
Leggat, W.; Marendy, E.M.; Baillie, B.; et al. Dinoflagellate symbiose: Strategies and adaptation for the acquisition and fixation of inorganic carbonic. Funct. Plant Biol. 2002, 29, 309‒322. https://doi.org/10.1071/pp01202.
- 96.
Fonseca, J.S.; Marangoni, L.F.B.; Marques, J.A.; et al. Elevated temperature and exposure to copper leads to changes in the antioxidant defense system of the reef-building coral Mussismilia harttii. Front. Physiol. 2021, 12, 804678. https://doi.org/10.3389/fphys.2021.804678.
- 97.
Fukushima, L.M., Fonseca, J.S.; Vidal, T.J.; et al. Impact of iron exposure on Brazilian coral reefs: Acute vs. chronic stress responses. Ecotoxicol. Environ. Saf. 2025, 298, 118309. https://doi.org/10.1016/j.ecoenv.2025.118309.
- 98.
Santini, O.; Chahbane, N.; Vasseur, P.; et al. Effects of low-level copper exposure on Ca2+-ATPase and carbonic anhydrase in the freshwater bivalve Anodonta anatina. Toxicol. Environ. Chem. 2011, 93, 1826–1837. https://doi.org/10.1080/02772240903217598.
- 99.
Prazeres, M.F.; Uthicke, S.; Pandolfi, J.M. Ocean acidification induces biochemical and morphological changes in the calcification process of large benthic foraminifera. Proc. R. Soc. B 2015, 282, 20142782. https://doi.org/10.1098/rspb.2014.2782.
- 100.
Viarengo, A.; Mancinelli, G.; Pertica, M.; et al. Effects of heavy metals on the Ca2+-ATPase activity present in gill cell plasma-membrane of mussels (Mytilus galloprovincialis Lam.). Comp. Biochem. Physiol. C 1993, 106, 655‒660. https://doi.org/10.1016/0742-8413(93)90223-8.
- 101.
Burlando, B.; Bonomo, M.; Capri, F.; et al. Different effects of Hg2+ and Cu2+ on mussel (Mytilus galloprovincialis) plasma membrane Ca2+-ATPase: Hg2+ induction of protein expression. Comp. Biochem. Physiol. C 2004, 139, 201‒207. https://doi.org/10.1016/j.cca.2004.11.001.
- 102.
Sousa, L.; Pessoa, M.T.C.; Costa, T.G.; et al. Iron overload impact on P-ATPases. Ann. Hematol. 2018, 97, 377‒385. https://doi.org/10.1007/s00277-017-3222-4.
- 103.
Leão, Z.M.A.N.; Kikuchi, R.K.P.; Testa, V. Corals and corals reefs of Brazil. In Latin America Coral Reefs; Cortes, J., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2003; pp. 9‒52. https://doi.org/10.1016/B978-044451388-5/50003-5.
- 104.
Mies, M.; Francini-Filho, R.B.; Zilberberg, C.; et al. South Atlantic coral reefs are major global warming refugia and less susceptible to bleaching. Front. Mar. Sci. 2020, 7, 514. https://doi.org/10.3389/fmars.2020.00514.
- 105.
Tunala, L.P.; Tâmega, F.T.; Duarte, H.M.; et al. Stress factors in the photobiology of the reef coral Siderastrea stellata. J. Exp. Mar. Bio. Ecol. 2019, 519, 151188. https://doi.org/10.1016/j.jembe.2019.151188.
- 106.
Longo, G.O.; Correia, L.F.; Mello, T.J. Coral recovery after a burial event: Insights on coral resilience in a marginal reef. Mar. Biodivers. 2020, 50, 92. https://doi.org/10.1007/s12526-020-01121-4.
- 107.
Pereira, P.H.; Lima, G.V.; Pontes, A.V.; et al. Unprecedented coral mortality on Southwestern Atlantic coral reefs following major thermal stress. Front. Mar. Sci. 2022, 9, 725778. https://doi.org/10.3389/fmars.2022.725778.
- 108.
Corazza, B.M.; Lacerda, C.H.; Güth, A.Z.; et al. No coral recovery three years after a major bleaching event in reefs in the Southwestern Atlantic refugium. Mar. Biol. 2024, 171, 114. https://doi.org/10.1007/s00227-024-04432-3.
- 109.
Mies, M.; Destri, G.; Lacerda, C.H.F.; et al. Coral bleaching and mortality across a 24° latitudinal range in the Southwestern Atlantic during the fourth global bleaching event. Coral Reefs 2025. https://doi.org/10.1007/s00338-025-02743-5.
- 110.
Mies, M.; Banha, T.N.S.; Francini-Filho, R.B.; et al. Reef ecosystems in the Brazilian continental shelf. In Oceanography, Biodiversity, Fisheries and Conservation of Brazilian Continental Shelf Habitats. Brazilian Marine Biodiversity; Sumida, P.Y.G., Mies, M., Eds.; Springer: Cham, Switzerland, 2025; pp. 115‒172. https://doi.org/10.1007/978-3-031-88338-5_5.
- 111.
Leão, Z.M.; Kikuchi, R.K.P.; Oliveira, M.D.M.; et al. Status of Eastern Brazilian coral reefs in time of climate changes. Pan-Am. J. Aquat. Sci. 2010, 5, 224‒235.
- 112.
Santana, E.F.C.; Mies, M.; Longo, G.O.; et al. Turbidity shapes shallow Southwestern Atlantic benthic reef communities. Mar. Environ. Res. 2023, 183, 105807. https://doi.org/10.1016/j.marenvres.2022.105807.
- 113.
Patrocinio, G.T.; Lopes, F.C.; Ciotti, Á.M.; et al. The Southwestern Atlantic coral Mussismilia hispida exhibit remarkable tolerance to nitrate enrichment. Coral Reefs 2025, 44, 629‒642. https://doi.org/10.1007/s00338-025-02631-y.
- 114.
Fonseca, J.S.; Mies, M.; Paranhos, A.; et al. Isolated and combined effects of thermal stress and copper exposure on the trophic behavior and oxidative status of the reef-building coral Mussismilia harttii. Environ. Pollut. 2021, 268, 115892. https://doi.org/10.1016/j.envpol.2020.115892.
- 115.
Tang, J.; Cai, W.; Yan, Z.; et al. Interactive effects of acidification and copper exposure on the reproduction and metabolism of coral endosymbiont Cladocopium goreaui. Mar. Pollut. Bull. 2022, 177, 113508. https://doi.org/10.1016/j.marpolbul.2022.113508.
- 116.
Cheng, M.; Luo, Y.; Yu, X.L.; et al. Effects of elevated temperature and copper exposure on the physiological state of the coral Galaxea fascicularis. Mar. Environ. Res. 2024, 193, 106218. https://doi.org/10.1016/j.marenvres.2023.106218.
- 117.
Cryer, S.E.; Schlosser, C.; Allison, N. The combined effects of ocean acidification and copper on the physiological responses of the tropical coral Stylophora pistillata. Mar. Environ. Res. 2022, 176, 105610. https://doi.org/10.1016/j.marenvres.2022.105610.
- 118.
Grillo, A.C.; Inagaki, K.Y.; Costa, P.G.; et al. Differential effects of iron enrichment on corals competing with macroalgae and zoantharians. Environ. Pollut. 2025, 371, 125944. https://doi.org/10.1016/j.envpol.2025.125944.
- 119.
Cui, L.; Cheng, C.; Li, X.; et al. Comprehensive assessment of copper’s effect on marine organisms under ocean acidification and warming in the 21st century. Sci. Total Environ. 2024, 927, 172145. https://doi.org/10.1016/j.scitotenv.2024.172145.
- 120.
Banc-Prandi, G.; Fine, M. Copper enrichment reduces thermal tolerance of the highly resistant Red Sea coral Stylophora pistillata. Coral Reefs 2019, 38, 285–296. https://doi.org/ 10.1007/s00338-019-01774-z.
- 121.
Hutchins, D.; Boyd, P. Marine phytoplankton and the changing ocean iron cycle. Nat. Clim. Chang. 2016, 6, 1072‒1079. https://doi.org/10.1038/nclimate3147.
- 122.
Altafim, G.L.; Alves, A.V.; Trevizani, T.H.; et al. Ocean warming and CO2-driven acidification can alter the toxicity of metal-contaminated sediments to the meiofauna community. Sci. Total Environ. 2023, 885, 163687. https://doi.org/10.1016/j.scitotenv.2023.163687.

This work is licensed under a Creative Commons Attribution 4.0 International License.


