The intrinsic limitations of cancer therapies promoted the development of safer liposomal nanocarriers capable of better distributing the payload away from normal tissues. Since then, liposomal nanocarriers have been considered the primary drug delivery system for many active pharmaceutical ingredients. These systems are now frequently investigated for the treatment of many infectious diseases. Along with the tremendous progress in the anticancer and antifungal liposomal nanomedicines, we have also gradually realised the difficulties associated with the existing liposomal nanocarrier designs. A better understanding of the nanocarrier-bio interactions may provide a new paradigm in liposomal nanocarrier design and better clinical endpoint efficacy. This short review focuses on the progress and benefits of two market-approved liposomal nanomedicines for cancer and fungal treatments.
- Open Access
- Review
Recent Clinical Successes in Liposomal Nanomedicines
- Wenjie Gu,
- Gavin P. Andrews,
- Yiwei Tian *
Author Information
Received: 19 Dec 2022 | Accepted: 26 Jan 2023 | Published: 03 Feb 2023
Abstract
Graphical Abstract
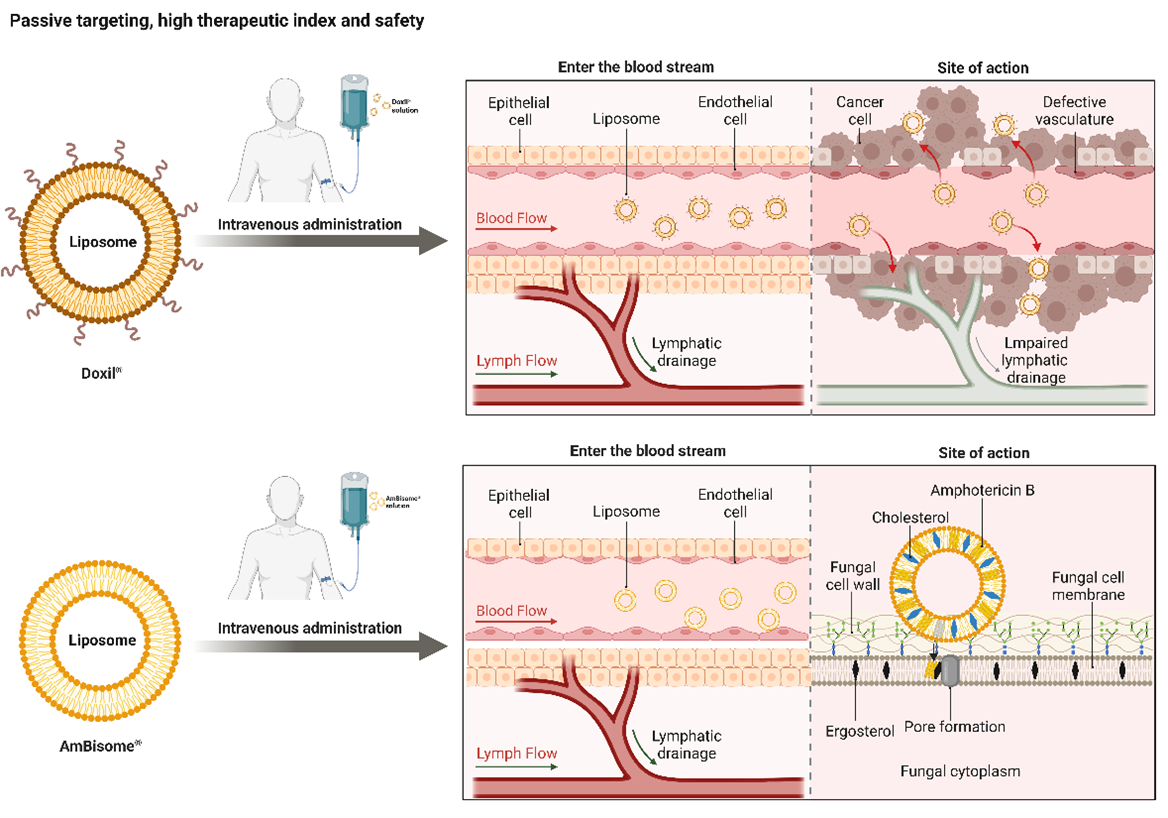
Keywords
liposome | nanomedicine | cancer | antifungal | nanocarriers | therapeutic | index | safety | efficacy
References
- 1.Ventola C.L. The nanomedicine revolution: part 2: current and future clinical applications. P&T, 2012, 37(10): 582-591.
- 2.Ventola C.L. Progress in nanomedicine: approved and investigational nanodrugs. P&T, 2017, 42(12): 742-755.
- 3.Wang Y.W.; Grainger D.W. Regulatory considerations specific to liposome drug development as complex drug products. Front. Drug. Deliv., 2022, 2: 901281.
- 4.Bangham A.D.; Standish M.M.; Watkins J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol., 1965, 13(1): 238-252.
- 5.Bulbake U.; Doppalapudi S.; Kommineni N.; et al. Liposomal formulations in clinical use: an updated review. Pharmaceutics, 2017, 9(2): 12.
- 6.Asadi K.; Gholami A. Virosome-based nanovaccines; a promising bioinspiration and biomimetic approach for preventing viral diseases: a review. Int. J. Biol. Macromol., 2021, 182: 648-658.
- 7.Gulati M.; Bajad S.; Singh S.; et al. Development of liposomal amphotericin B formulation. J. Microencapsulation, 1998, 15(2): 137-151.
- 8.Liu P.; Chen G.L.; Zhang J.C. A review of liposomes as a drug delivery system: current status of approved products, regulatory environments, and future perspectives. Molecules, 2022, 27(4): 1372.
- 9.Bozzuto G.; Molinari A. Liposomes as nanomedical devices. Int. J. Nanomed., 2015, 10: 975-999.
- 10.Beltrán-Gracia E.; López-Camacho A.; Higuera-Ciapara I.; et al. Nanomedicine review: clinical developments in liposomal applications. Cancer Nanotechnol., 2019, 10(1): 11.
- 11.Hou X.C.; Zaks T.; Langer R.; et al. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater., 2021, 6(12): 1078-1094.
- 12.Kisby T.; Yilmazer A.; Kostarelos K. Reasons for success and lessons learnt from nanoscale vaccines against COVID-19. Nat. Nanotechnol., 2021, 16(8): 843-850.
- 13.Akinc A.; Maier M.A.; Manoharan M.; et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol, 2019, 14(12): 1084-1087.
- 14.He H., Yuan D.F.; Wu Y.; et al. Pharmacokinetics and Pharmacodynamics modeling and simulation systems to support the development and regulation of liposomal drugs. Pharmaceutics, 2019, 11(3): 110.
- 15.Boafo G.F.; Thapa K.T.; Ekpo M.D.; et al. The role of cryoprotective agents in liposome stabilization and preservation. Int. J. Mol. Sci., 2022, 23(20): 12487.
- 16.Li Z.L.; Zhang Y.L.; Wurtz W.; et al. Characterization of nebulized liposomal amikacin (Arikace) as a function of droplet size. J. Aerosol Med. Pulm. Drug Delivery, 2008, 21(3): 245-254.
- 17.LLS Health Technical Team. Excipients for solubility enhancement: enabling oral and injectable formulations. (accessed on 18 January 2023). https://www.lubrizol.com/Health/Blog/2022/03/Excipients-for-Solubility-Enhancement---Enabling-Oral-and-Injectable-Formulations.
- 18.Savjani K.T.; Gajjar A.K.; Savjani J.K. Drug solubility: importance and enhancement techniques. ISRN Pharm., 2012, 2012: 195727.
- 19.He H.S.; Lu Y.; Qi J.P.; et al. Adapting liposomes for oral drug delivery. Acta Pharm. Sin. B, 2019, 9(1): 36-48.
- 20.Giardiello M.; Liptrott N.J.; McDonald T.O.; et al. Accelerated oral nanomedicine discovery from miniaturized screening to clinical production exemplified by paediatric HIV nanotherapies. Nat. Commun., 2016, 7: 13184.
- 21.Bakshi R.P.; Tatham L.M.; Savage A.C.; et al. Long-acting injectable atovaquone nanomedicines for malaria prophylaxis. Nat. Commun., 2018, 9(1): 315.
- 22.World Health Organization. Cancer. (accessed on 2 December 2022). https://www.who.int/health-topics/cancer#tab=tab_3.
- 23.Carvalho C.; Santos R.X.; Cardoso S.; et al. Doxorubicin: the good, the bad and the ugly effect. Curr. Med. Chem., 2009, 16(25): 3267-3285.
- 24.Brown J.R.; Imam S.H. 5 recent studies on doxorubicin and its analogues. Prog. Med. Chem., 1985, 21: 169-236.
- 25.Yingchoncharoen P.; Kalinowski D.S.; Richardson D.R. Lipid-based drug delivery systems in cancer therapy: what is available and what is yet to come. Pharmacol. Rev., 2016, 68(3): 701-787.
- 26.Chang H.I.; Yeh M.K. Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. Int. J. Nanomed., 2012, 7: 49-60.
- 27.O’Bryan R.M.; Luce J.K.; Talley R.W.; et al. Phase II evaluation of adriamycin in human neoplasia. Cancer, 1973, 32(1): 1-8.
- 28.Lefrak E.A.; Piťha J.; Rosenheim S.; et al. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer, 1973, 32(2): 302-314.
- 29.Chlebowski R.T. Adriamycin (doxorubicin) cardiotoxicity: a review. West. J. Med., 1979, 131(5): 364-368.
- 30.Cancer Chemotherapy Reports. U.S. department of health, education, and welfare, public health service, national institutes of health; 1975.
- 31.Park K. The beginning of the end of the nanomedicine hype. J. Controlled Release, 2019, 305: 221-222.
- 32.Hortobágyi G.N. Anthracyclines in the treatment of cancer. An overview. Drugs, 1997, 54(4): 1-7.
- 33.Waterhouse D.N.; Tardi P.G.; Mayer L.D.; et al. A comparison of liposomal formulations of doxorubicin with drug administered in free form: changing toxicity profiles. Drug Saf., 2001, 24(12): 903-920.
- 34.Birtle A.J. Anthracyclines and cardiotoxicity. Clin. Oncol., 2000, 12(3): 146-152.
- 35.Rivankar S. An overview of doxorubicin formulations in cancer therapy. J. Cancer Res. Ther., 2014, 10(4): 853-858.
- 36.Anon. Celsion Announces Enrollment Completion Pivotal Phase III OPTIMA Study of ThermoDox® in Primary Liver Cancer–Duke OTC. (accessed on 12 December 2022). https://otc.duke.edu/news/celsion-announces-enrollment-completion-pivotal-phase-iii-optima-study-of-thermodox-in-primary-liver-cancer/.
- 37.Petersen G.H.; Alzghari S.K.; Chee W.; et al. Meta-analysis of clinical and preclinical studies comparing the anticancer efficacy of liposomal versus conventional non-liposomal doxorubicin. J. Controlled Release, 2016, 232: 255-264.
- 38.Morigi V.; Tocchio A.; Bellavite C.B.; et al. Nanotechnology in medicine: from inception to market domination. J. Drug Delivery, 2012, 2012: 389485.
- 39.Bharali D.J.; Mousa S.A. Emerging nanomedicines for early cancer detection and improved treatment: current perspective and future promise. Pharmacol. Ther., 2010, 128(2): 324-335.
- 40.Seigneuric R.; Markey L.; Nuyten D.S.A.; et al. From nanotechnology to nanomedicine: applications to cancer research. Curr. Mol. Med., 2010, 10(7): 640-652.
- 41.Rafiyath S.M.; Rasul M.; Lee B.; et al. Comparison of safety and toxicity of liposomal doxorubicin vs. conventional anthracyclines: a meta-analysis. Exp. Hematol. Oncol., 2012, 1(1): 10.
- 42.Safra T. Cardiac safety of liposomal anthracyclines. The Oncologist, 2003, 8(S2): 17-24.
- 43.Šimůnek T.; Štěrba M.; Popelová O.; et al. Anthracycline-induced cardiotoxicity: overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol. Rep., 2009, 61(1): 154-171.
- 44.Allen T.M.; Mumbengegwi D.R.; Charrois G.J.R. Anti-CD19-targeted liposomal doxorubicin improves the therapeutic efficacy in murine B-cell lymphoma and ameliorates the toxicity of liposomes with varying drug release rates. Clin. Cancer Res., 2005, 11(9): 3567-3573.
- 45.Safra T.; Muggia F.; Jeffers S.; et al. Pegylated liposomal doxorubicin (doxil): reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann. Oncol., 2000, 11(8): 1029-1033.
- 46.Kesterson J.P.; Odunsi K.; Lele S. High cumulative doses of pegylated liposomal doxorubicin are not associated with cardiac toxicity in patients with gynecologic malignancies. Chemotherapy, 2010, 56(2): 108-111.
- 47.Fukuda A.; Tahara K.; Hane Y.; et al. Comparison of the adverse event profiles of conventional and liposomal formulations of doxorubicin using the FDA adverse event reporting system. PLoS One, 2017, 12(9): e0185654.
- 48.Northfelt D.W.; Dezube B.J.; Thommes J.A.; et al. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi’s sarcoma: results of a randomized phase III clinical trial. J. Clin. Oncol., 1998, 16(7): 2445-2451.
- 49.O’Brien M.E.R.; Wigler N.; Inbar M.; et al. Reduced cardiotoxicity and comparable efficacy in a phase IIItrial of pegylated liposomal doxorubicin HCl(CAELYX™/Doxil®) versus conventional doxorubicin forfirst-line treatment of metastatic breast cancer. Ann. Oncol., 2004, 15(3): 440-449.
- 50.Swenson C.E.; Perkins W.R.; Roberts P.; et al. Liposome technology and the development of Myocet™ (liposomal doxorubicin citrate). The Breast, 2001, 10, Supplement 2: 1-7.
- 51.Anon. FDA fast track designation for myocet for metastatic breast cancer. Oncology Times, 2010, 32(3): 24.
- 52.Immordino M.L.; Dosio F.; Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed., 2006, 1(3): 297-315.
- 53.Schnyder A.; Huwyler J. Drug transport to brain with targeted liposomes. NeuroRX, 2005, 2(1): 99-107.
- 54.Shao K.; Hou Q.S.; Duan W.; et al. Intracellular drug delivery by sulfatide-mediated liposomes to gliomas. J. Controlled Release, 2006, 115(2): 150-157.
- 55.Saul J.M.; Annapragada A.; Natarajan J.V.; et al. Controlled targeting of liposomal doxorubicin via the folate receptor in vitro. J. Controlled Release, 2003, 92(1/2): 49-67.
- 56.Itokazu M.; Kumazawa S.; Wada E.; et al. Sustained release of adriamycin from implanted hydroxyapatite blocks for the treatment of experimental osteogenic sarcoma in mice. Cancer Lett., 1996, 107(1): 11-18.
- 57.Bromberg L.; Alakhov V. Effects of polyether-modified poly(acrylic acid) microgels on doxorubicin transport in human intestinal epithelial Caco-2 cell layers. J. Controlled Release, 2003, 88(1): 11-22.
- 58.Zocchi E.; Tonetti M.; Polvani C.; et al. Encapsulation of doxorubicin in liver-targeted erythrocytes increases the therapeutic index of the drug in a murine metastatic model. Proc. Natl. Acad. Sci. U. S. A., 1989, 86(6): 2040-2044.
- 59.Olivier J.C. Drug transport to brain with targeted nanoparticles. NeuroRX, 2005, 2(1): 108-119.
- 60.Petri B.; Bootz A.; Khalansky A.; et al. Chemotherapy of brain tumour using doxorubicin bound to surfactant-coated poly (butyl cyanoacrylate) nanoparticles: revisiting the role of surfactants. J. Controlled Release, 2007, 117(1): 51-58.
- 61.Gaffi. Improving outcomes for patients with fungal infections across the world a road map for the next decade. (accessed on 15 December 2022). https://gaffi.org/wp-content/uploads/GAFFI_Road_Map_interactive-final0415.pdf.
- 62.Rodrigues M.L.; Nosanchuk J.D. Fungal diseases as neglected pathogens: a wake-up call to public health officials. PLoS Neglected Trop. Dis., 2020, 14(2): e0007964.
- 63.Raut A.; Huy N.T. Rising incidence of mucormycosis in patients with COVID-19: another challenge for India amidst the second wave? Lancet Respir. Med., 2021, 9(8): e77.
- 64.Hoenigl M. Invasive fungal disease complicating coronavirus disease 2019: when it rains, it spores. Clin. Infect. Dis., 2021, 73(7): e1645-e1648.
- 65.Rivnay B.; Wakim J.; Avery K.; et al. Critical process parameters in manufacturing of liposomal formulations of amphotericin B. Int. J. Pharm., 2019, 565: 447-457.
- 66.Gallis H.A.; Drew R.H.; Pickard W.W. Amphotericin B: 30 years of clinical experience. Rev. Infect. Dis., 1990, 12(2): 308-329.
- 67.Drugs.com. Fungizone prescribing information. (accessed on 12 December 2022). https://www.drugs.com/pro/fungizone.html.
- 68.Min Y.Z.; Caster J.M.; Eblan M.J.; et al. Clinical translation of nanomedicine. Chem. Rev., 2015, 115(19): 11147-11190.
- 69.Wingard J.R.; Kubilis P.; Lee L.; et al. Clinical significance of nephrotoxicity in patients treated with amphotericin B for suspected or proven aspergillosis. Clin. Infect. Dis., 1999, 29(6): 1402-1407.
- 70.Heinemann V.; Bosse D.; Jehn U.; et al. Pharmacokinetics of liposomal amphotericin B (Ambisome) in critically ill patients. Antimicrob. Agents Chemother., 1997, 41(6): 1275-1280.
- 71.Jarvis J.N.; Lawrence D.S.; Meya D.B.; et al. Single-dose liposomal amphotericin B treatment for cryptococcal meningitis. N. Engl. J. Med., 2022, 386(12): 1109-1120.
- 72.Rodrigues A.V.; Valério-Bolas A.; Alexandre-Pires G.; et al. Zoonotic visceral leishmaniasis: new insights on innate immune response by blood macrophages and liver Kupffer cells to Leishmania infantum parasites. Biology, 2022, 11(1): 100.
- 73.Boswell G.W.; Buell D.; Bekersky I. AmBisome (liposomal amphotericin B): a comparative review. J. Clin. Pharmacol., 1998, 38(7): 583-592.
- 74.Wingard J.R.; White M.H.; Anaissie E.; et al. A randomized, double-blind comparative trial evaluating the safety of liposomal amphotericin B versus amphotericin B lipid complex in the empirical treatment of febrile neutropenia. L Amph/ABLC Collaborative Study Group. Clin. Infect. Dis., 2000, 31(5): 1155-1163.
- 75.Lestner J.; McEntee L.; Johnson A.; et al. Experimental models of short courses of liposomal amphotericin B for induction therapy for cryptococcal meningitis. Antimicrob. Agents Chemother., 2017, 61(6): e00090-17.
- 76.Walsh T.J.; Yeldandi V.; McEvoy M.; et al. Safety, tolerance, and pharmacokinetics of a small unilamellar liposomal formulation of amphotericin B (AmBisome) in neutropenic patients. Antimicrob. Agents Chemother., 1998, 42(9): 2391-2398.
- 77.McLintock L.A.; Cook G.; Holyoake T.L.; et al. High loading dose AmBisome is efficacious and well tolerated in the management of invasive fungal infection in hematology patients. Haematologica, 2007, 92(4): 572-573.
- 78.World Health Organization. New guidelines from WHO recommend a simpler, safer treatment for cryptococcal disease in people living with HIV. (accessed on 14 December 2022). https://www.who.int/news/item/20-04-2022-rapid-advice-new-guidelines-for-simpler-safer-treatment-for-cryptococcal-disease-in-plhiv.
- 79.Meyerhoff A. U.S. food and drug administration approval of AmBisome (liposomal amphotericin B) for treatment of visceral leishmaniasis. Clin. Infect. Dis., 1999, 28(1): 42-48.
- 80.Sundar S.; Chakravarty J. Liposomal amphotericin B and leishmaniasis: dose and response. J. Global Infect. Dis., 2010, 2(2): 159-166.
- 81.MSF Access Campaign. Open letter to Gilead and Viatris: we need affordable treatments for the rise of serious invasive fungal diseases. (accessed on 25 January 2023). https://msfaccess.org/open-letter-ambisome-Gilead.
How to Cite
Gu, W.; Andrews, G. P.; Tian, Y. Recent Clinical Successes in Liposomal Nanomedicines. International Journal of Drug Discovery and Pharmacology 2023, 2 (1), 52–59. https://doi.org/10.53941/ijddp.0201009.
RIS
BibTex
Copyright & License

Wenjie Gu, Gavin P. Andrews, Yiwei Tian
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


