Tempeh, a traditional Indonesian food, is made through fermentation of soybean and increases the bioavailability of beneficial nutrients, including phytoestrogens and protein. Recent research indicates that fermenting soybeans to make tempeh could improve bio functional properties including anticancer activity. This study aims to explore whether defatted soybeans and tempeh (fermented soybeans) extracts possess anti-proliferative activity of human colorectal (CRC) cancer cells. The defatted soybean and tempeh samples were extracted at a concentration of 35 g/100 mL using 70% ethanol, evaporated and then lyophilized. HCT116 cells were treated with soybean extract (SE) and tempeh extract (TE) for 24 h and MTT (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) assay, flow cytometry, and Western blot analyses were performed. SE and TE exhibited inhibitory effects on cell viability, with TE showing a more significant dose-dependent inhibition compared to SE. Cell cycle analysis showed a significant increase in G1 arrest, along with a significant decrease in S and G2/M phases in both SE- and TE-treated cells. The induction of apoptosis was observed in cells treated with both SE and TE. Additionally, Western blot analysis showed increased Poly (ADP-ribose) polymerase cleavage for both treatments, indicating activation of apoptotic pathways in CRC cells treated with SE and TE. These findings indicate that soybeans and tempeh may be effective dietary options to help prevent colorectal cancer.
- Open Access
- Article
Antiproliferative Activity of Soybean and Tempeh Extracts in Human Colorectal Cancer Cells
- Rongjie Fan 1,†,
- Yong Hoon Joo 1,†,
- Made Astawan 2,
- Dmitriy Smolensky 3,
- Seong-Ho Lee 1,*,
- Cheng-I Wei 1,*
Author Information
Received: 22 Sep 2025 | Revised: 16 Dec 2025 | Accepted: 08 Jan 2026 | Published: 19 Jan 2026
Abstract
Graphical Abstract
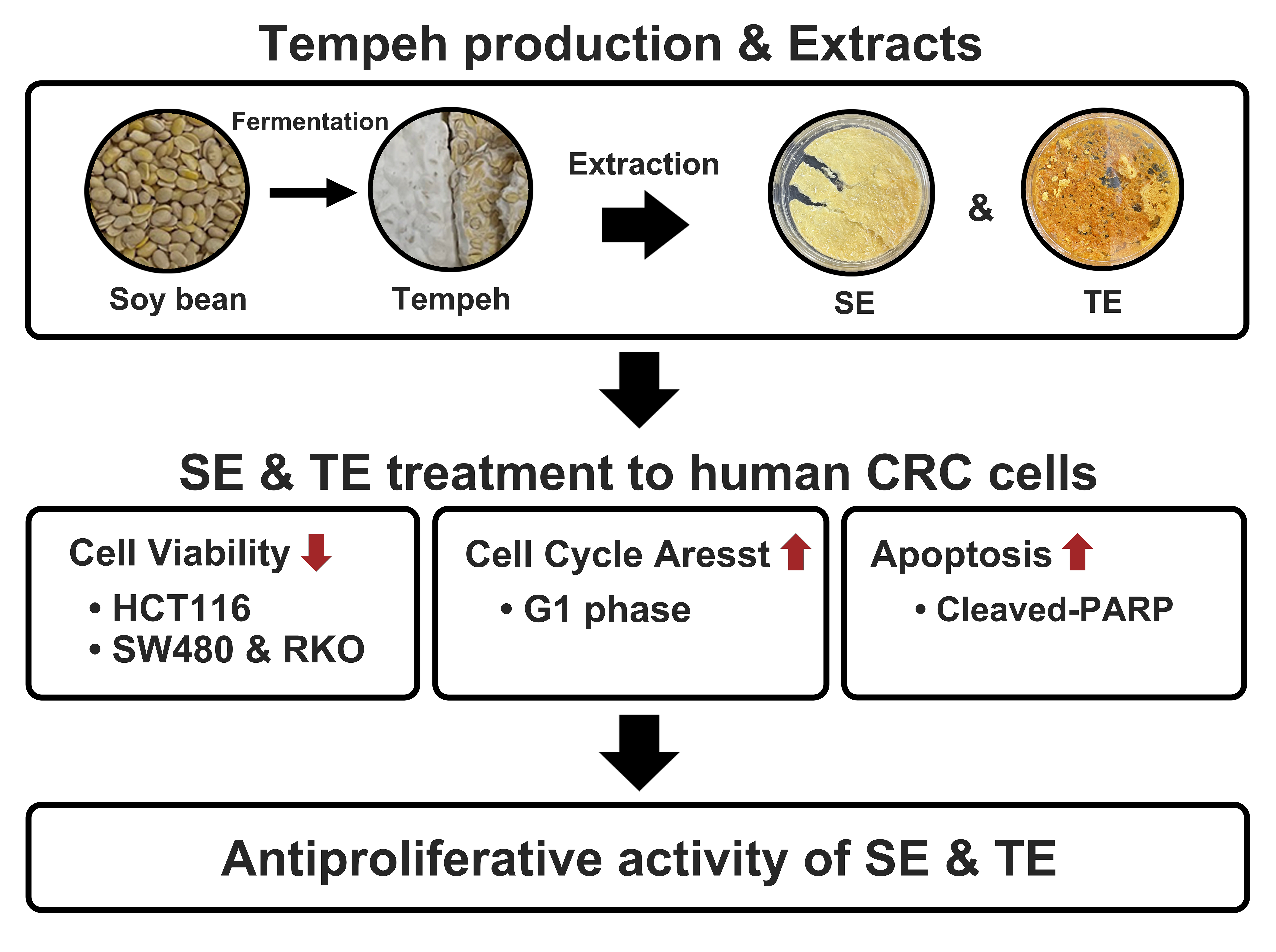
Keywords
soybeans | tempeh | fermentation | anticancer activity | colorectal cancer
References
- 1.
Collado, M.; Castillo, M.; Muñoz de Mier, G.J.; et al. The Diet as a Modulator of Tumor Microenvironment in Colorectal Cancer Patients. Int. J. Mol. Sci. 2023, 24, 7317. https://doi.org/10.3390/ijms24087317.
- 2.
He, K.; Gan, W.J. Wnt/β-Catenin Signaling Pathway in the Development and Progression of Colorectal Cancer. Cancer Manag. Res. 2023, 15, 435–448. https://doi.org/10.2147/CMAR.S411168.
- 3.
Kaushik, S.; Shyam, H.; Sharma, R.; et al. Dietary isoflavone daidzein synergizes centchroman action via induction of apoptosis and inhibition of PI3K/Akt pathway in MCF-7/MDA MB-231 human breast cancer cells. Phytomedicine 2018, 40, 116–124. https://doi.org/10.1016/j.phymed.2018.01.007.
- 4.
Su, H.K.; Chen, W.C.; Lu, J.H.; et al. The effects of using Tempeh as a supplement for type 2 diabetes. Food Sci. Nutr. 2023, 11, 3339–3347. https://doi.org/10.1002/fsn3.3319.
- 5.
Gardner, C.D.; Messina, M.; Lawson, L.D.; et al. Soy, garlic, and ginkgo biloba: Their potential role in cardiovascular disease prevention and treatment. Curr. Atheroscler. Rep. 2003, 5, 468–475. https://doi.org/10.1007/s11883-003-0037-7.
- 6.
Ahmad, A.; Ramasamy, K.; Jaafar, S.M.; et al. Total isoflavones from soybean and tempeh reversed scopolamine-induced amnesia, improved cholinergic activities and reduced neuroinflammation in brain. Food Chem. Toxicol. 2014, 65, 120–128. https://doi.org/10.1016/j.fct.2013.12.025.
- 7.
Astawan, M.; Cahyani, A.P.; Wresdiyati, T. Antioxidant activity and isoflavone content of overripe Indonesian tempe. Food Res. 2023, 7, 42–50. https://doi.org/10.26656/fr.2017.7(S1).16.
- 8.
Ahmad, A.; Ramasamy, K.; Majeed, A.B.; et al. Enhancement of β-secretase inhibition and antioxidant activities of tempeh, a fermented soybean cake through enrichment of bioactive aglycones. Pharm. Biol. 2015, 53, 758–766. https://doi.org/10.3109/13880209.2014.942791.
- 9.
Kim, I.S. Current perspectives on the beneficial effects of soybean isoflavones and their metabolites on plants. Food Sci. Biotechnol. 2022, 31, 515–526. https://doi.org/10.1007/s10068-022-01070-7.
- 10.
Kuligowski, M.; Jasińska-Kuligowska, I.; Nowak, J. Evaluation of bean and soy tempeh influence on intestinal bacteria and estimation of antibacterial properties of bean tempeh. Pol. J. Microbiol. 2013, 62, 189–194.
- 11.
Hwang, J.H.; Wu, S.J.; Wu, P.L.; et al. Neuroprotective effect of tempeh against lipopolysaccharide-induced damage in BV-2 microglial cells. Nutr. Neurosci. 2019, 22, 840–849. https://doi.org/10.1080/1028415X.2018.1456040.
- 12.
Divate, N.R.; Ardanareswari, K. Effects of Soybean and Tempeh Water Extracts on Regulation of Intestinal Flora and Prevention of Colon Precancerous Lesions in Rats. Processes 2023, 11, 257. https://doi.org/10.3390/pr11010257.
- 13.
Tamia, G.; Amarakoon, D.; Wei, C.-I.; et al. Evaluation of Humulus lupulus (hops) compound anti-proliferative properties with mechanisms in human colorectal cancer cells. Food Biosci. 2024, 57, 103493.
- 14.
Legesse Bedada, T.; Feto, T.K.; Awoke, K.S.; et al. Probiotics for cancer alternative prevention and treatment. Biomed. Pharmacother. 2020, 129, 110409. https://doi.org/10.1016/j.biopha.2020.110409.
- 15.
Cichońska, P.; Kowalska, E.; Ziarno, M. The Survival of Psychobiotics in Fermented Food and the Gastrointestinal Tract: A Review. Microorganisms 2023, 11, 996. https://doi.org/10.3390/microorganisms11040996.
- 16.
Elhalis, H.; Chin, X.H.; Chow, Y. Soybean fermentation: Microbial ecology and starter culture technology. Crit. Rev. Food Sci. Nutr. 2023, 64, 7648–7670. https://doi.org/10.1080/10408398.2023.2188951.
- 17.
Wang, W.; Dia, V.P.; Vasconez, M.; et al. Analysis of soybean protein-derived peptides and the effect of cultivar, environmental conditions, and processing on lunasin concentration in soybean and soy products. J. AOAC Int. 2008, 91, 936–946.
- 18.
Liu, W.-T.; Huang, C.-L.; Liu, R.; et al. Changes in isoflavone profile, antioxidant activity, and phenolic contents in Taiwanese and Canadian soybeans during tempeh processing. LWT 2023, 186, 115207.
- 19.
Chatterjee, C.; Gleddie, S.; Xiao, C.W. Soybean Bioactive Peptides and Their Functional Properties. Nutrients 2018, 10, 1211. https://doi.org/10.3390/nu10091211.
- 20.
Athaillah, Z.A.; Muzdalifah, D.; Lestari, A.; et al. Phenolic Compound Profile and Functionality of Aqueous Overripe Tempe Extracts. Curr. Res. Nutr. Food Sci. J. 2019, 7, 382–392. https://doi.org/10.12944/CRNFSJ.7.2.08.
- 21.
Lukman, K.; Nugraha, P.; Taslim, N.A.; et al. Identification of novel anti-tumor peptides from enzymatic hydrolysis of soy-based tempeh and their mechanism in pancreatic and colorectal cancer cells. J. Agric. Food Res. 2025, 22, 102084.
- 22.
Rahaiee, S.; Assadpour, E.; Faridi Esfanjani, A.; et al. Application of nano/microencapsulated phenolic compounds against cancer. Adv. Colloid. Interface Sci. 2020, 279, 102153. https://doi.org/10.1016/j.cis.2020.102153.
- 23.
Sharifi-Rad, J.; Quispe, C.; Imran, M.; et al. Genistein: An integrative overview of its mode of action, pharmacological properties, and health benefits. Oxidative Med. Cell. Longev. 2021, 2021, 3268136.
- 24.
Fang, M.Z.; Chen, D.; Sun, Y.; et al. Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy. Clin. Cancer Res. 2005, 11, 7033–7041. https://doi.org/10.1158/1078-0432.CCR-05-0406.
- 25.
Li, M.; Zhang, Z.; Hill, D.L.; et al. Genistein, a dietary isoflavone, down-regulates the MDM2 oncogene at both transcriptional and posttranslational levels. Cancer Res. 2005, 65, 8200–8208. https://doi.org/10.1158/0008-5472.CAN-05-1302.
- 26.
Touny, L.H.; Banerjee, P.P. Identification of both Myt-1 and Wee-1 as necessary mediators of the p21-independent inactivation of the cdc-2/cyclin B1 complex and growth inhibition of TRAMP cancer cells by genistein. Prostate 2006, 66, 1542–1555. https://doi.org/10.1002/pros.20495.
- 27.
Zhou, J.; Li, L.U.; Fang, L.I.; et al. Quercetin reduces cyclin D1 activity and induces G1 phase arrest in HepG2 cells. Oncol. Lett. 2016, 12, 516–522. https://doi.org/10.3892/ol.2016.4639.
- 28.
Ronghe, A.; Chatterjee, A.; Singh, B.; et al. Differential regulation of estrogen receptors α and β by 4-(E)-(4-hydroxyphenylimino)-methylbenzene,1,2-diol, a novel resveratrol analog. J. Steroid Biochem. Mol. Biol. 2014, 144, 500–512. https://doi.org/10.1016/j.jsbmb.2014.09.015.
- 29.
Shafiee, G.; Saidijam, M.; Tayebinia, H.; et al. Beneficial effects of genistein in suppression of proliferation, inhibition of metastasis, and induction of apoptosis in PC3 prostate cancer cells. Arch. Physiol. Biochem. 2022, 128, 694–702. https://doi.org/10.1080/13813455.2020.1717541.
- 30.
Pabona, J.M.; Dave, B.; Su, Y.; et al. The soybean peptide lunasin promotes apoptosis of mammary epithelial cells via induction of tumor suppressor PTEN: Similarities and distinct actions from soy isoflavone genistein. Genes. Nutr. 2013, 8, 79–90. https://doi.org/10.1007/s12263-012-0307-5.
- 31.
Lule, V.K.; Garg, S.; Pophaly, S.D.; et al. Potential health benefits of lunasin: A multifaceted soy-derived bioactive peptide. J. Food Sci. 2015, 80, R485–R494. https://doi.org/10.1111/1750-3841.12786.
- 32.
Kim, S.E.; Kim, H.H.; Kim, J.Y.; et al. Anticancer activity of hydrophobic peptides from soy proteins. Biofactors 2000, 12, 151–155. https://doi.org/10.1002/biof.5520120124.
- 33.
Choi, Y.H.; Zhang, L.; Lee, W.; et al. Genistein-induced G2/M arrest is associated with the inhibition of cyclin B1 and the induction of p21 in human breast carcinoma cells. Int. J. Oncol. 1998, 13, 391–397.
- 34.
Yin-Ching, C. Tempeh attenuates cognitive deficit, antioxidant imbalance, and amyloid β of senescence-accelerated mice by modulating Nrf2 expression via MAPK pathway. J. Funct. Foods 2018, 50, 112–119. https://doi.org/10.1016/j.jff.2018.09.023.
- 35.
Su, S.-J.; Chow, N.-H.; Kung, M.-L.; et al. Effects of soy isoflavones on apoptosis induction and G2-M arrest in human hepatoma cells involvement of caspase-3 activation, Bcl-2 and Bcl-XL downregulation, and Cdc2 kinase activity. Nutr. Cancer 2003, 45, 113–123.
- 36.
An, S.-Y.; An, H.-K.; Kim, K.-S.; et al. Induction of autophagy by oleifolioside A in HCT-116 human colorectal cancer cells. Appl. Biol. Chem. 2023, 66, 32.

This work is licensed under a Creative Commons Attribution 4.0 International License.


