AMP-activated protein kinase (AMPK) is a key enzyme broadly involved in regulating cellular metabolism, often called an “energy sensor”. Activated AMPK promotes ATP production and storage within cells, primarily by inhibiting ATP-consuming anabolic processes (such as protein, lipid, and ribosomal synthesis) and initiating ATP-producing catabolic pathways (such as fatty acid oxidation and glycolysis) to maintain energy homeostasis. AMPK regulates metabolic processes in various peripheral tissues, including glucose and lipid metabolism, cholesterol metabolism, and fatty acid and protein metabolism in pancreatic β-cells, the cardiovascular system, liver, kidneys, skeletal muscles, and the central nervous system. As an antidiabetic drug, the multi-organ protective effects of Glucagon-like peptide-1 receptor agonists (GLP-1RA) are increasingly being recognized. This paper reviews the mechanisms by which GLP-1RA confers organ protection via the AMPK signaling pathway.
- Open Access
- Review
AMPK-Mediated Multi-Organ Protective Effects of GLP-1 Receptor Agonists
- Xin Wang 1,
- Linxi Wang 2, *
Author Information
Received: 11 Oct 2024 | Revised: 23 Oct 2024 | Accepted: 20 Dec 2024 | Published: 09 Jan 2025
Abstract
Graphical Abstract
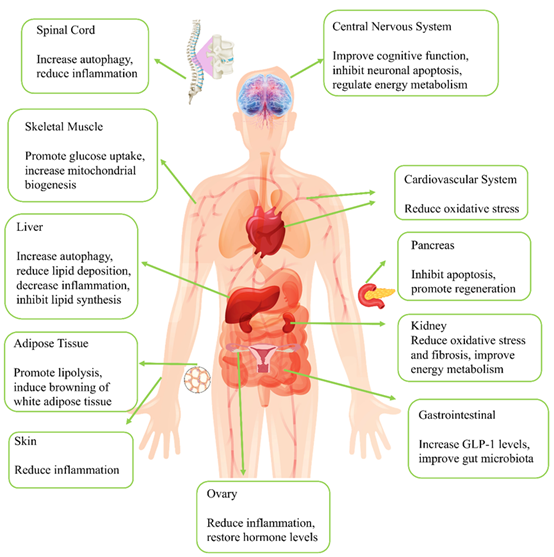
Keywords
References
- 1.Sébastien, H.; Reuben, J.S. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2017, 19, 121–135. https://doi.org/10.1038/nrm.2017.95.
- 2.Carling, D. AMPK signalling in health and disease. Curr. Opin. Cell Biol. 2017, 45, 31–37. https://doi.org/10.1016/j.ceb.2017.01.005.
- 3.Olga, G.; Franziska, K.; Mark, H.R. Metabolic control by AMPK in white adipose tissue. Trends Endocrinol. Metab. 2023, 34, 704–717. https://doi.org/10.1016/j.tem.2023.08.011.
- 4.Muhammad, A.; Yury, L. Emerging Role of cAMP/AMPK Signaling. Cells 2022, 11, 308. https://doi.org/10.3390/cells11020308.
- 5.Xiaodi, Z.; Yi, Z.; Dafen, G.; et al. Exercise Improves Heart Function after Myocardial Infarction: The Merits of AMPK. Cardiovasc. Drugs Ther. 2024, 5, 1–9. https://doi.org/10.1007/s10557-024-07564-2.
- 6.Xiaorui, Y.; Ziyuan, G.; Chunli, S. AMPK, a key molecule regulating aging-related myocardial ischemia-reperfusion injury. Mol. Biol. Rep. 2024, 51, 257. https://doi.org/10.1007/s11033-023-09050-8.
- 7.Seyed Zanyar, A.; Fereshteh, F.; Rana, K.; et al. AMPK Signaling Pathway as a Potential Therapeutic Target for Parkinson’s Disease. Adv. Pharm. Bull. 2024, 14, 120. https://doi.org/10.34172/apb.2024.013.
- 8.Luning, Y.; Di, L.; Shide, J.; et al. SIRT1 signaling pathways in sarcopenia: Novel mechanisms and potential therapeutic targets. Biomed. Pharmacother. 2024, 177, 116917. https://doi.org/10.1016/j.biopha.2024.116917.
- 9.Qianxia, H.; Yingcong, R.; Ping, Y.; et al. Targeting the AMPK/Nrf2 Pathway: A Novel Therapeutic Approach for Acute Lung Injury. J. Inflamm. Res. 2024, 17, 4683–4700. https://doi.org/10.2147/jir.S467882.
- 10.Kosuke, Y.; Mako, Y.-Y.; Shinji, K. A novel therapeutic target for kidney diseases: Lessons learned from starvation response. Pharmacol. Ther. 2024, 254, 108590. https://doi.org/10.1016/j.pharmthera.2024.108590.
- 11.Gaoyi, R.; Fangquan, W.; Dibang, S.; et al. Metformin: Update on mechanisms of action on liver diseases. Front. Nutr. 2024, 10, 1327814. https://doi.org/10.3389/fnut.2023.1327814.
- 12.American Diabetes Association. Obesity Management for the Treatment of Type 2 Diabetes. Diabetes Care 2020, 43, S89–S97. https://doi.org/10.2337/dc20-S008.
- 13.Alfaris, N.; Waldrop, S.; Johnson, V.; et al. GLP-1 single, dual, and triple receptor agonists for treating type 2 diabetes and obesity: A narrative review. EClinicalMedicine 2024, 75, 102782. https://doi.org/10.1016/j.eclinm.2024.102782.
- 14.Tina, K.T.; Richard, P.; Juris, J.M. Efficacy, safety and cardiovascular outcomes of once-daily oral semaglutide in patients with type 2 diabetes: The PIONEER programme. Diabetes Obes. Metab. 2020, 22, 1263–1277. https://doi.org/10.1111/dom.14054.
- 15.Christopher, S.; Shin-Ichi, H.; George, M.T.; et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): A double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017, 5, 251–260. https://doi.org/10.1016/s2213-8587(17)30013-x.
- 16.Johannes, F.E.M.; David, D.Ø.; Kirstine, B.-F.; et al. Liraglutide and Renal Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 839–848.
- 17.John, B.B.; Daniel, J.D.; Kristin, L.T.; et al. DURATION-1: Exenatide once weekly produces sustained glycemic control and weight loss over 52 weeks. Diabetes Care 2010, 33, 1255–1261. https://doi.org/10.2337/dc09-1914.
- 18.Katherine, R.T.; Mark, C.L.; Brian, R.; et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): A multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018, 6, 605–617. https://doi.org/10.1016/s2213-8587(18)30104-9.
- 19.Fatemeh, T.; Rosaria Anna, F.; Lucia, S.; et al. Bridging the gap between GLP1-receptor agonists and cardiovascular outcomes: Evidence for the role of tirzepatide. Cardiovasc. Diabetol. 2024, 23, 242. https://doi.org/10.1186/s12933-024-02319-7.
- 20.John, D.; Subodh, V.; Benjamin, M.S.; et al. Semaglutide and cardiovascular outcomes in patients with obesity and prevalent heart failure: A prespecified analysis of the SELECT trial. Lancet 2024, 404, 773–786. https://doi.org/10.1016/s0140-6736(24)01498-3.
- 21.Steven, P.M.; Gilbert, H.D.; Kirstine, B.-F.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. https://doi.org/10.1056/NEJMoa1603827.
- 22.Drucker, D. The benefits of GLP-1 drugs beyond obesity. Science 2024, 385, 258–260. https://doi.org/10.1126/science.adn4128.
- 23.David, M.W.; Asif, N.; Marc, E. Renal Outcomes in Type 2 Diabetes: A Review of Cardiovascular and Renal Outcome Trials. Diabetes Ther. 2019, 11, 369–386. https://doi.org/10.1007/s13300-019-00747-3.
- 24.Ammon, J.; Jackson, C. Semaglutide, Obesity-Related Heart Failure, and Type 2 Diabetes. N. Engl. J. Med. 2024, 391, 381. https://doi.org/10.1056/NEJMc2406233.
- 25.Kosiborod, M.; Abildstrøm, S.; Borlaug, B.; et al. Semaglutide in Patients with Heart Failure with Preserved Ejection Fraction and Obesity. N. Engl. J. Med. 2023, 389, 1069–1084. https://doi.org/10.1056/NEJMoa2306963.
- 26.Courcoulas, A.; Patti, M.; Hu, B.; et al. Long-Term Outcomes of Medical Management vs Bariatric Surgery in Type 2 Diabetes. JAMA 2024, 331, 654–664. https://doi.org/10.1001/jama.2024.0318.
- 27.Sachs, S.; Bastidas-Ponce, A.; Tritschler, S.; et al. Targeted pharmacological therapy restores beta-cell function for diabetes remission. Nat. Metab. 2020, 2, 192–209. https://doi.org/10.1038/s42255-020-0171-3.
- 28.Bergmann, N.; Davies, M.; Lingvay, I.; et al. Semaglutide for the treatment of overweight and obesity: A review. Diabetes Obes. Metab. 2023, 25, 18–35. https://doi.org/10.1111/dom.14863.
- 29.Wadden, T.; Chao, A.; Machineni, S.; et al. Tirzepatide after intensive lifestyle intervention in adults with overweight or obesity: The SURMOUNT-3 phase 3 trial. Nat. Med. 2023, 29, 2909–2918. https://doi.org/10.1038/s41591-023-02597-w.
- 30.Melson, E.; Ashraf, U.; Papamargaritis, D.; et al. What is the pipeline for future medications for obesity? Int. J. Obes. 2024, 1, 1–19. https://doi.org/10.1038/s41366-024-01473-y.
- 31.Wali, Z.; Hattiwale, S.; Jamal, A.; et al. GLP-1/Sigma/RAGE receptors: An evolving picture of Alzheimer’s disease pathology and treatment. Ageing Res. Rev. 2024, 93, 102134. https://doi.org/10.1016/j.arr.2023.102134.
- 32.Jiang, H.; Zang, L. GLP-1/GLP-1RAs: New Options for the Drug Treatment of NAFLD. Curr. Pharm. Des. 2024, 30, 100–114. https://doi.org/10.2174/0113816128283153231226103218.
- 33.Logan, K.T.; Gregory, R.S. AMPK and the Endocrine Control of Metabolism. Endocr. Rev. 2023, 44, 910–933. https://doi.org/10.1210/endrev/bnad012.
- 34.Thomas, M.; Nikooienejad, A.; Bray, R.; et al. Dual GIP and GLP-1 Receptor Agonist Tirzepatide Improves Beta-cell Function and Insulin Sensitivity in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2021, 106, 388–396. https://doi.org/10.1210/clinem/dgaa863.
- 35.Miao, X.Y.; Gu, Z.Y.; Liu, P.; et al. The human glucagon-like peptide-1 analogue liraglutide regulates pancreatic beta-cell proliferation and apoptosis via an AMPK/mTOR/P70S6K signaling pathway. Peptides 2013, 39, 71–79. https://doi.org/10.1016/j.peptides.2012.10.006.
- 36.Wang, K.; Sun, Y.; Lin, P.; et al. Liraglutide Activates AMPK Signaling and Partially Restores Normal Circadian Rhythm and Insulin Secretion in Pancreatic Islets in Diabetic Mice. Biol. Pharm. Bull. 2015, 38, 1142–1149. https://doi.org/10.1248/bpb.b15-00024.
- 37.Chang, T.J.; Tseng, H.C.; Liu, M.W.; et al. Glucagon-like peptide-1 prevents methylglyoxal-induced apoptosis of beta cells through improving mitochondrial function and suppressing prolonged AMPK activation. Sci. Rep. 2016, 6, 23403. https://doi.org/10.1038/srep23403.
- 38.Kim, Y.K.; Park, J.H.; Park, S.H.; et al. Protective role of glucagon-like peptide-1 against glucosamine-induced cytotoxicity in pancreatic beta cells. Cell Physiol. Biochem. 2010, 25, 211–220. https://doi.org/10.1159/000276555.
- 39.Tajima, K.; Shirakawa, J.; Togashi, Y.; et al. AMPK is involved in the regulation of incretin receptors expression in pancreatic islets under a low glucose concentration. PLoS ONE 2013, 8, e64633. https://doi.org/10.1371/journal.pone.0064633.
- 40.Li, R.; Sun, X.; Li, P.; et al. GLP-1-Induced AMPK Activation Inhibits PARP-1 and Promotes LXR-Mediated ABCA1 Expression to Protect Pancreatic β-Cells Against Cholesterol-Induced Toxicity Through Cholesterol Efflux. Front. Cell Dev. Biol. 2021, 9, 646113. https://doi.org/10.3389/fcell.2021.646113.
- 41.Wharton, S.; Batterham, R.L.; Bhatta, M.; et al. Two-year effect of semaglutide 2.4 mg on control of eating in adults with overweight/obesity: STEP 5. Obesity 2023, 31, 703–715. https://doi.org/10.1002/oby.23673.
- 42.Sayers, S.R.; Reimann, F.; Gribble, F.M.; et al. Proglucagon Promoter Cre-Mediated AMPK Deletion in Mice Increases Circulating GLP-1 Levels and Oral Glucose Tolerance. PLoS ONE 2016, 11, e0149549. https://doi.org/10.1371/journal.pone.0149549.
- 43.Zeng, R.; Zeng, Y.; Wang, Q.; et al. Sleeve gastrectomy decreased hepatic lipid accumulation by inducing autophagy via AMPK/mTOR pathway. Biochem. Biophys. Res. Commun. 2023, 653, 115–125. https://doi.org/10.1016/j.bbrc.2023.02.071.
- 44.Wei, L.; Yalin, Z.; Han, S.; et al. Goat Milk Improves Glucose Homeostasis via Enhancement of Hepatic and Skeletal Muscle AMP-Activated Protein Kinase Activation and Modulation of Gut Microbiota in Streptozocin-Induced Diabetic Rats. Mol. Nutr. Food Res. 2021, 65, 2000888. https://doi.org/10.1002/mnfr.202000888.
- 45.Koychev, I.; Reid, G.; Nguyen, M.; et al. Inflammatory proteins associated with Alzheimer’s disease reduced by a GLP1 receptor agonist: A post hoc analysis of the EXSCEL randomized placebo controlled trial. Alzheimer’s Res. Ther. 2024, 16, 212. https://doi.org/10.1186/s13195-024-01573-x.
- 46.Hurtado-Carneiro, V.; Sanz, C.; Roncero, I.; et al. Glucagon-like peptide 1 (GLP-1) can reverse AMP-activated protein kinase (AMPK) and S6 kinase (P70S6K) activities induced by fluctuations in glucose levels in hypothalamic areas involved in feeding behaviour. Mol. Neurobiol. 2012, 45, 348–361. https://doi.org/10.1007/s12035-012-8239-z.
- 47.Burmeister, M.A.; Ayala, J.; Drucker, D.J.; et al. Central glucagon-like peptide 1 receptor-induced anorexia requires glucose metabolism-mediated suppression of AMPK and is impaired by central fructose. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E677–E685. https://doi.org/10.1152/ajpendo.00446.2012.
- 48.Lopez, M.; Dieguez, C.; Nogueiras, R. Hypothalamic GLP-1: The control of BAT thermogenesis and browning of white fat. Adipocyte 2015, 4, 141–145. https://doi.org/10.4161/21623945.2014.983752.
- 49.Palleria, C.; Leo, A.; Andreozzi, F.; et al. Liraglutide prevents cognitive decline in a rat model of streptozotocin-induced diabetes independently from its peripheral metabolic effects. Behav. Brain Res. 2017, 321, 157–169. https://doi.org/10.1016/j.bbr.2017.01.004.
- 50.Ma, D.; Liu, X.; Liu, J.; et al. Long-term liraglutide ameliorates nigrostriatal impairment via regulating AMPK/PGC-1a signaling in diabetic mice. Brain Res. 2019, 1714, 126–132. https://doi.org/10.1016/j.brainres.2019.02.030.
- 51.El-Sayed, S.; Ali, S.; Ibrahim, W. Potential neuroprotective and autophagy-enhancing effects of alogliptin on lithium/pilocarpine-induced seizures in rats: Targeting the AMPK/SIRT1/Nrf2 axis. Life Sci. 2024, 352, 122917. https://doi.org/10.1016/j.lfs.2024.122917.
- 52.Liu, Y.; Hu, Z.; Wang, J.; et al. Puerarin alleviates depressive-like behaviors in high-fat diet-induced diabetic mice via modulating hippocampal GLP-1R/BDNF/TrkB signaling. Nutr. Neurosci. 2023, 26, 997–1010. https://doi.org/10.1080/1028415x.2022.2112439.
- 53.Marso, S.; Bain, S.; Consoli, A.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. New Engl. J. Med. 2016, 375, 1834–1844. https://doi.org/10.1056/NEJMoa1607141.
- 54.Lincoff, A.; Brown-Frandsen, K.; Colhoun, H.; et al. Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. New Engl. J. Med. 2023, 389, 2221–2232. https://doi.org/10.1056/NEJMoa2307563.
- 55.Balteau, M.; Van Steenbergen, A.; Timmermans, A.D.; et al. AMPK activation by glucagon-like peptide-1 prevents NADPH oxidase activation induced by hyperglycemia in adult cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1120-1133,. https://doi.org/10.1152/ajpheart.00210.2014.
- 56.Inoue, T.; Inoguchi, T.; Sonoda, N.; et al. GLP-1 analog liraglutide protects against cardiac steatosis, oxidative stress and apoptosis in streptozotocin-induced diabetic rats. Atherosclerosis 2015, 240, 250–259. https://doi.org/10.1016/j.atherosclerosis.2015.03.026.
- 57.Ma, G.; Liu, Y.; Wang, Y.; et al. Liraglutide reduces hyperglycemia-induced cardiomyocyte death through activating glucagon-like peptide 1 receptor and targeting AMPK pathway. J. Recept. Signal Transduct. Res. 2020, 40, 133–140. https://doi.org/10.1080/10799893.2020.1719517.
- 58.Zhou, Y.; He, X.; Chen, Y.; et al. Exendin-4 attenuates cardiac hypertrophy via AMPK/mTOR signaling pathway activation. Biochem. Biophys. Res. Commun. 2015, 468, 394–399. https://doi.org/10.1016/j.bbrc.2015.09.179.
- 59.Wang, J.; Fan, S.; Xiong, Q.; et al. Glucagon-like peptide-1 attenuates cardiac hypertrophy via the AngII/AT1R/ACE2 and AMPK/mTOR/p70S6K pathways. Acta Biochim. Et. Biophys. Sin. 2021, 53, 1189–1197. https://doi.org/10.1093/abbs/gmab099.
- 60.Fang, B.; Liu, F.; Yu, X.; et al. Liraglutide alleviates myocardial ischemia‒reperfusion injury in diabetic mice. Mol. Cell. Endocrinol. 2023, 572, 111954. https://doi.org/10.1016/j.mce.2023.111954.
- 61.Xie, S.; Zhang, M.; Shi, W.; et al. Long-Term Activation of Glucagon-like peptide-1 receptor by Dulaglutide Prevents Diabetic Heart Failure and Metabolic Remodeling in Type 2 Diabetes. J. Am. Heart Assoc. 2022, 11, e026728. https://doi.org/10.1161/jaha.122.026728.
- 62.Rodríguez, C.; Muñoz, M.; Contreras, C.; et al. AMPK, metabolism, and vascular function. FEBS J. 2021, 288, 3746–3771. https://doi.org/10.1111/febs.15863.
- 63.Hattori, Y.; Jojima, T.; Tomizawa, A.; et al. A glucagon-like peptide-1 (GLP-1) analogue, liraglutide, upregulates nitric oxide production and exerts anti-inflammatory action in endothelial cells. Diabetologia 2010, 53, 2256–2263. https://doi.org/10.1007/s00125-010-1831-8.
- 64.Koska, J.; Sands, M.; Burciu, C.; et al. Exenatide Protects Against Glucose- and Lipid-Induced Endothelial Dysfunction: Evidence for Direct Vasodilation Effect of GLP-1 Receptor Agonists in Humans. Diabetes 2015, 64, 2624–2635. https://doi.org/10.2337/db14-0976.
- 65.Wei, R.; Ma, S.; Wang, C.; et al. Exenatide exerts direct protective effects on endothelial cells through the AMPK/Akt/eNOS pathway in a GLP-1 receptor-dependent manner. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E947–E957. https://doi.org/10.1152/ajpendo.00400.2015.
- 66.Li, N.; Zhao, Y.; Yue, Y.; et al. Liraglutide ameliorates palmitate-induced endothelial dysfunction through activating AMPK and reversing leptin resistance. Biochem. Biophys. Res. Commun. 2016, 478, 46–52. https://doi.org/10.1016/j.bbrc.2016.07.095.
- 67.Tang, S.T.; Su, H.; Zhang, Q.; et al. Sitagliptin inhibits endothelin-1 expression in the aortic endothelium of rats with streptozotocin-induced diabetes by suppressing the nuclear factor-kappaB/IkappaBalpha system through the activation of AMP-activated protein kinase. Int. J. Mol. Med. 2016, 37, 1558–1566. https://doi.org/10.3892/ijmm.2016.2578.
- 68.Tang, S.T.; Zhang, Q.; Tang, H.Q.; et al. Effects of glucagon-like peptide-1 on advanced glycation endproduct-induced aortic endothelial dysfunction in streptozotocin-induced diabetic rats: Possible roles of Rho kinase- and AMP kinase-mediated nuclear factor kappaB signaling pathways. Endocrine 2016, 53, 107–116. https://doi.org/10.1007/s12020-015-0852-y.
- 69.Krasner, N.M.; Ido, Y.; Ruderman, N.B.; et al. Glucagon-like peptide-1 (GLP-1) analog liraglutide inhibits endothelial cell inflammation through a calcium and AMPK dependent mechanism. PLoS ONE 2014, 9, e97554. https://doi.org/10.1371/journal.pone.0097554.
- 70.Batchuluun, B.; Inoguchi, T.; Sonoda, N.; et al. Metformin and liraglutide ameliorate high glucose-induced oxidative stress via inhibition of PKC-NAD(P)H oxidase pathway in human aortic endothelial cells. Atherosclerosis 2014, 232, 156–164. https://doi.org/10.1016/j.atherosclerosis.2013.10.025.
- 71.Wang, Y.G.; Yang, T.L. Liraglutide reduces oxidized LDL-induced oxidative stress and fatty degeneration in Raw 264.7 cells involving the AMPK/SREBP1 pathway. J. Geriatr. Cardiol. JGC 2015, 12, 410–416. https://doi.org/10.11909/j.issn.1671-5411.2015.04.013.
- 72.Chai, W.; Fu, Z.; Aylor, K.W.; et al. Liraglutide prevents microvascular insulin resistance and preserves muscle capillary density in high-fat diet-fed rats. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E640-648,. https://doi.org/10.1152/ajpendo.00205.2016.
- 73.Taguchi, K.; Bessho, N.; Kaneko, N.; et al. Glucagon-like peptide-1 increased the vascular relaxation response via AMPK/Akt signaling in diabetic mice aortas. Eur. J. Pharmacol. 2019, 865, 172776. https://doi.org/10.1016/j.ejphar.2019.172776.
- 74.Chen, K.; Jin, H.; Wu, Z.; et al. Glucagon-like peptide-1 receptor agonist exendin 4 ameliorates diabetes-associated vascular calcification by regulating mitophagy through the AMPK signaling pathway. Mol. Med. (Camb. Mass.) 2024, 30, 58. https://doi.org/10.1186/s10020-024-00817-8.
- 75.Baek, C.; Kim, H.; Moon, S.; et al. Liraglutide, a glucagon-like peptide-1 receptor agonist, induces ADAM10-dependent ectodomain shedding of RAGE via AMPK activation in human aortic endothelial cells. Life Sci. 2022, 292, 120331. https://doi.org/10.1016/j.lfs.2022.120331.
- 76.Han, F.; Hou, N.; Liu, Y.; et al. Liraglutide improves vascular dysfunction by regulating a cAMP-independent PKA-AMPK pathway in perivascular adipose tissue in obese mice. Biomed. Pharmacother. 2019, 120, 109537. https://doi.org/10.1016/j.biopha.2019.109537.
- 77.Al-Damry, N.T.; Attia, H.A.; Al-Rasheed, N.M.; et al. Sitagliptin attenuates myocardial apoptosis via activating LKB-1/AMPK/Akt pathway and suppressing the activity of GSK-3beta and p38alpha/MAPK in a rat model of diabetic cardiomyopathy. Biomed. Pharmacother. 2018, 107, 347–358. https://doi.org/10.1016/j.biopha.2018.07.126.
- 78.Ding, Y.; Peng, Y.; Wu, H.; et al. The protective roles of liraglutide on Kawasaki disease via AMPK/mTOR/NF-κB pathway. Int. Immunopharmacol. 2023, 117, 110028. https://doi.org/10.1016/j.intimp.2023.110028.
- 79.Gastaldelli, A.; Cusi, K.; Fernández Landó, L.; et al. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): A substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet. Diabetes Endocrinol. 2022, 10, 393–406. https://doi.org/10.1016/s2213-8587(22)00070-5.
- 80.Zhou, J.; Poudel, A.; Chandramani-Shivalingappa, P.; et al. Liraglutide induces beige fat development and promotes mitochondrial function in diet induced obesity mice partially through AMPK-SIRT-1-PGC1-alpha cell signaling pathway. Endocrine 2019, 64, 271–283. https://doi.org/10.1007/s12020-018-1826-7.
- 81.Beiroa, D.; Imbernon, M.; Gallego, R.; et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 2014, 63, 3346–3358. https://doi.org/10.2337/db14-0302.
- 82.Fan, Y.; Xia, M.; Yan, H.; et al. Efficacy of beinaglutide in the treatment of hepatic steatosis in type 2 diabetes patients with nonalcoholic fatty liver disease: A randomized, open-label, controlled trial. Diabetes Obes. Metab. 2024, 26, 772–776. https://doi.org/10.1111/dom.15359.
- 83.Zhang, J.M.; Sun, Y.S.; Zhao, L.Q.; et al. SCFAs-Induced GLP-1 Secretion Links the Regulation of Gut Microbiome on Hepatic Lipogenesis in Chickens. Front. Microbiol. 2019, 10, 2176. https://doi.org/10.3389/fmicb.2019.02176.
- 84.Lee, M.Y.; Chen, W.C.; Hsu, W.H.; et al. Liraglutide Inhibits Hepatitis C Virus Replication Through an AMP Activated Protein Kinase Dependent Mechanism. Int. J. Mol. Sci. 2019, 20, 4569. https://doi.org/10.3390/ijms20184569.
- 85.Ben-Shlomo, S.; Zvibel, I.; Shnell, M.; et al. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J. Hepatol. 2011, 54, 1214–1223. https://doi.org/10.1016/j.jhep.2010.09.032.
- 86.He, Q.; Sha, S.; Sun, L.; et al. GLP-1 analogue improves hepatic lipid accumulation by inducing autophagy via AMPK/mTOR pathway. Biochem. Biophys. Res. Commun. 2016, 476, 196–203. https://doi.org/10.1016/j.bbrc.2016.05.086.
- 87.Yu, P.; Xu, X.; Zhang, J.; et al. Liraglutide Attenuates Nonalcoholic Fatty Liver Disease through Adjusting Lipid Metabolism via SHP1/AMPK Signaling Pathway. Int. J. Endocrinol. 2019, 2019, 1567095. https://doi.org/10.1155/2019/1567095.
- 88.Zhao, P.; Sun, X.; Chaggan, C.; et al. An AMPK-caspase-6 axis controls liver damage in nonalcoholic steatohepatitis. Science 2020, 367, 652–660. https://doi.org/10.1126/science.aay0542.
- 89.Guo, T.; Yan, W.; Cui, X.; et al. Liraglutide attenuates type 2 diabetes mellitus-associated non-alcoholic fatty liver disease by activating AMPK/ACC signaling and inhibiting ferroptosis. Mol. Med. 2023, 29, 132. https://doi.org/10.1186/s10020-023-00721-7.
- 90.Reis-Barbosa, P.; Marcondes-de-Castro, I.; Marinho, T.; et al. The mTORC1/AMPK pathway plays a role in the beneficial effects of semaglutide (GLP-1 receptor agonist) on the liver of obese mice. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101922. https://doi.org/10.1016/j.clinre.2022.101922.
- 91.Perkovic, V.; Tuttle, K.; Rossing, P.; et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. New Engl. J. Med. 2024, 391, 109–121. https://doi.org/10.1056/NEJMoa2403347.
- 92.Rui, S.; Songyan, Q.; Yunhui, L.; et al. GLP-1 receptor agonist attenuates tubular cell ferroptosis in diabetes via enhancing AMPK-fatty acid metabolism pathway through macropinocytosis. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167060. https://doi.org/10.1016/j.bbadis.2024.167060.
- 93.Xu, W.W.; Guan, M.P.; Zheng, Z.J.; et al. Exendin-4 alleviates high glucose-induced rat mesangial cell dysfunction through the AMPK pathway. Cell Physiol. Biochem. 2014, 33, 423–432. https://doi.org/10.1159/000358623.
- 94.Wang, L.; Chen, Z.; Liu, X.; et al. GLP-1 Receptor Agonist Improves Mitochondrial Energy Status and Attenuates Nephrotoxicity In Vivo and In Vitro. Metabolites 2023, 13, 1121. https://doi.org/10.3390/metabo13111121.
- 95.Han, L.; Chen, X.; Wan, D.; et al. One anastomosis gastric bypass ameliorates diabetic nephropathy via regulating the GLP-1-mediated Sirt1/AMPK/PGC1α pathway. Clin. Exp. Nephrol. 2024, 28, 1051–1061. https://doi.org/10.1007/s10157-024-02516-4.
- 96.Ye, K.; Zhao, Y.; Huang, W.; et al. Sodium butyrate improves renal injury in diabetic nephropathy through AMPK/SIRT1/PGC-1α signaling pathway. Sci. Rep. 2024, 14, 17867. https://doi.org/10.1038/s41598-024-68227-8.
- 97.Xuan, Y.; Ding, T.; Mao, X.; et al. Liraglutide alleviates high-fat diet-induced kidney injury in mice by regulating the CaMKKβ/AMPK pathway. Ren. Fail. 2024, 46, 2351473. https://doi.org/10.1080/0886022x.2024.2351473.
- 98.Guo, C.; Ye, F.; Jian, Y.; et al. MicroRNA-214-5p aggravates sepsis-related acute kidney injury in mice. Drug Dev. Res. 2022, 83, 339–350. https://doi.org/10.1002/ddr.21863.
- 99.Liu, M.; Guo, S.; Li, X.; et al. Semaglutide Alleviates Ovary Inflammation via the AMPK/SIRT1/NF-κB Signaling Pathway in Polycystic Ovary Syndrome Mice. Drug Des. Dev. Ther. 2024, 18, 3925–3938. https://doi.org/10.2147/dddt.S484531.
- 100.Ligumsky, H.; Amir, S.; Arbel Rubinstein, T.; et al. Glucagon-like peptide-1 analogs activate AMP kinase leading to reversal of the Warburg metabolic switch in breast cancer cells. Med. Oncol. 2024, 41, 138. https://doi.org/10.1007/s12032-024-02390-w.
- 101.Julie, R.L.; Charlotte, J.; Simon, B.K.J.; et al. Healthy Weight Loss Maintenance with Exercise, Liraglutide, or Both Combined. N. Engl. J. Med. 2021, 384, 1719–1730. https://doi.org/10.1056/NEJMoa2028198.
- 102.Wu, L.; Zhou, M.; Li, T.; et al. GLP-1 regulates exercise endurance and skeletal muscle remodeling via GLP-1R/AMPK pathway. Biochim. Et. Biophys. Acta. Mol. Cell Res. 2022, 1869, 119300. https://doi.org/10.1016/j.bbamcr.2022.119300.
- 103.Li, Z.; Ni, C.L.; Yao, Z.; et al. Liraglutide enhances glucose transporter 4 translocation via regulation of AMP-activated protein kinase signaling pathways in mouse skeletal muscle cells. Metabolism 2014, 63, 1022–1030. https://doi.org/10.1016/j.metabol.2014.05.008.
- 104.Andreozzi, F.; Raciti, G.A.; Nigro, C.; et al. The GLP-1 receptor agonists exenatide and liraglutide activate Glucose transport by an AMPK-dependent mechanism. J. Transl. Med. 2016, 14, 229. https://doi.org/10.1186/s12967-016-0985-7.
- 105.Xu, F.; Cao, H.; Chen, Z.; et al. Short-term GLP-1 receptor agonist exenatide ameliorates intramyocellular lipid deposition without weight loss in ob/ob mice. Int. J. Obes. 2020, 44, 937–947. https://doi.org/10.1038/s41366-019-0513-y.
- 106.Cao, H.Y.; Xu, F.; Chen, Z.L.; et al. [Effect of exendin-4 on lipid deposition in skeletal muscle of diet-induced obese mice and its underlying mechanism]. Zhonghua Yi Xue Za Zhi 2017, 97, 131–136. https://doi.org/10.3760/cma.j.issn.0376-2491.2017.02.011.
- 107.Shoier, N.; Ghareib, S.; Kothayer, H.; et al. Vitamin D3 mitigates myopathy and metabolic dysfunction in rats with metabolic syndrome: The potential role of dipeptidyl peptidase-4. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 10, 1–19. https://doi.org/10.1007/s00210-024-03439-3.
- 108.Zhang, D.; Yu, D.; Mei, X.; et al. Liraglutide provides neuroprotection by regulating autophagy through the AMPK-FOXO3 signaling pathway in a spinal contusion injury rat model. Neurosci. Lett. 2020, 720, 134747. https://doi.org/10.1016/j.neulet.2020.134747.
- 109.Song, S.; Guo, R.; Mehmood, A.; et al. Liraglutide attenuate central nervous inflammation and demyelination through AMPK and pyroptosis-related NLRP3 pathway. CNS Neurosci. Ther. 2022, 28, 422–434. https://doi.org/10.1111/cns.13791.
- 110.Karacabeyli, D.; Lacaille, D. Glucagon-Like Peptide 1 Receptor Agonists in Patients With Inflammatory Arthritis or Psoriasis: A Scoping Review. J. Clin. Rheumatol. Pract. Rep. Rheum. Musculoskelet. Dis. 2024, 30, 26–31. https://doi.org/10.1097/rhu.0000000000001949.
- 111.Yang, J.; Wang, Z.; Zhang, X. GLP-1 receptor agonist impairs keratinocytes inflammatory signals by activating AMPK. Exp. Mol. Pathol. 2019, 107, 124–128. https://doi.org/10.1016/j.yexmp.2019.01.014.
How to Cite
Wang, X.; Wang, L. AMPK-Mediated Multi-Organ Protective Effects of GLP-1 Receptor Agonists. Health and Metabolism 2025, 2 (1), 4. https://doi.org/10.53941/hm.2025.100004.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


