Photodynamic therapy is a novel clinical treatment for malignant tumors, and has recently been extended to anti-bacteria and anti-virus applications. Phthalocyanine photosensitizers possess good photosensitization properties, but their high hydrophobicity and lack of targeting capabilities limit their application. By conjugating a pentalysine peptidyl moiety to hydrophobic phthalocyanine, a novel photosensitizer (ZnPc(Lys)5) was synthesized. This review systematically summarizes the design, development, safety and characterization of ZnPc(Lys)5, and describes its applications and mechanisms in anti-tumor, anti-bacterial and anti-viral areas, as well as exploring its prospects for applications beyond photodynamic therapy. This review demonstrates that the peptide conjugation is an effective strategy for enhancing water solubility and broadens the applications of phthalocyanine-type photosensitizers. Furthermore, the importance of dynamic balance between the monomeric and aggregate conformations of phthalocyanine underlying its photodynamic effect is highlighted, providing a fresh perspective for the design and application of photosensitizers.
- Open Access
- Review
A Simple Phthalocyanine-Peptide Conjugate as Targeting Photosensitizer and Its Broad Applications in Health
- Zheng Chen 1,
- Jincan Chen 2,
- Dafeng Liu 3,
- Jingyi Chen 1,
- Linlin Li 1,
- Dan Chen 1,
- Naisheng Chen 1,
- Jinling Huang 1,
- Zhuo Chen 2,
- Peng Xu 4,
- Longguang Jiang 1,
- Cai Yuan 4, *,
- Yunbin Jiang 5, *,
- Mingdong Huang 1, *
Author Information
Received: 28 Aug 2024 | Revised: 09 Oct 2024 | Accepted: 17 Oct 2024 | Published: 25 Oct 2024
Abstract
Graphical Abstract
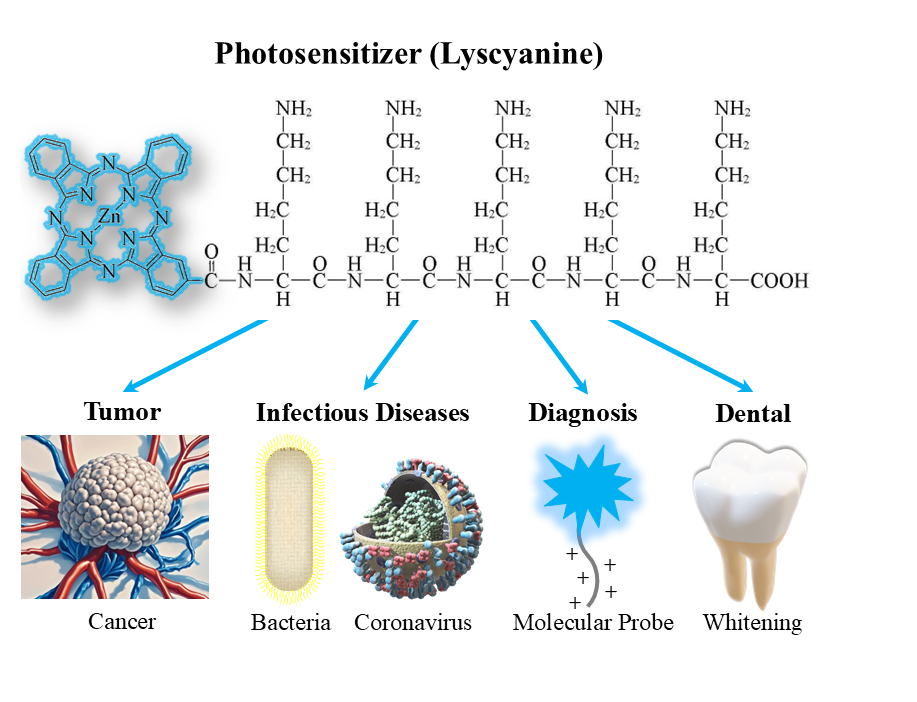
Keywords
photosensitizer | photodynamic therapy | targeting | anti-tumor | antibacterial | monomer-aggregate dynamics
References
- 1.Van Straten, D.; Mashayekhi, V.; De Bruijn, H.; Oliveira, S.; Robinson, D. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9, 19. https://doi.org/10.3390/cancers9020019.
- 2.Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. https://doi.org/10.3322/caac.20114.
- 3.Li, X.S.; Lovell, J.F.; Yoon, J.; Chen, X.Y. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. https://doi.org/10.1038/s41571-020-0410-2.
- 4.Fenner, B.J.; Cheung, C.M.G.; Sim, S.S.; Lee, W.K.; Staurenghi, G.; Lai, T.Y.Y.; Ruamviboonsuk, P.; Kokame, G.; Yanagi, Y.; Teo, K.Y.C. Evolving treatment paradigms for PCV. Eye 2021, 36, 257–265. https://doi.org/10.1038/s41433-021-01688-7.
- 5.Ito, Y.; Ikeda, Y.; Iizuka, H. Cellulitis due to gas‐producing organism with niveau formation. J. Dermatol. 2009, 36, 622–623. https://doi.org/10.1111/j.1346-8138.2009.00713.x.
- 6.Xiao, Q.; Mai, B.; Nie, Y.; Yuan, C.; Xiang, M.; Shi, Z.; Wu, J.; Leung, W.; Xu, C.; Yao, S.Q.; et al. In Vitro and In Vivo Demonstration of Ultraefficient and Broad-Spectrum Antibacterial Agents for Photodynamic Antibacterial Chemotherapy. Acs Appl Mater Inter 2021, 13, 11588–11596. https://doi.org/10.1021/acsami.0c20837.
- 7.Salmon-Divon, M.; Nitzan, Y.; Malik, Z. Mechanistic aspects of Escherichia coli photodynamic inactivation by cationic tetra-meso(N-methylpyridyl)porphine. Photochem Photobiol Sci 2004, 3, 423–429. https://doi.org/10.1039/b315627n.
- 8.Monro, S.; Colon, K.L.; Yin, H.; Roque, J., 3rd; Konda, P.; Gujar, S.; Thummel, R.P.; Lilge, L.; Cameron, C.G.; McFarland, S.A. Transition Metal Complexes and Photodynamic Therapy from a Tumor-Centered Approach: Challenges, Opportunities, and Highlights from the Development of TLD1433. Chem Rev 2018, 119, 797–828. https://doi.org/10.1021/acs.chemrev.8b00211.
- 9.Cai, L.Z.; Huang, M.D.; Chen, Z. Research progress of antitumor photosensitizers. J. Fujian Med. Univ. 2013, 121–126. (In Chinese).
- 10.Josefsen, L.B.; Boyle, R.W. Photodynamic therapy: Novel third-generation photosensitizers one step closer? Brit J Pharmacol 2008, 154, 1–3. https://doi.org/10.1038/bjp.2008.98.
- 11.Alzeibak, R.; Mishchenko, T.A.; Shilyagina, N.Y.; Balalaeva, I.V.; Vedunova, M.V.; Krysko, D.V. Targeting immunogenic cancer cell death by photodynamic therapy: Past, present and future. J. Immunother. Cancer 2021, 9, e001926. https://doi.org/10.1136/jitc-2020-001926.
- 12.Galstyan, A. Turning Photons into Drugs: Phthalocyanine-Based Photosensitizers as Efficient Photoantimicrobials. Chemistry 2021, 27, 1903–1920. https://doi.org/10.1002/chem.202002703.
- 13.Chen, J.C.; Chen, H.W.; Li, Y.D.; Wang, J.D.; Chen, N.S.; Huang, J.L.; Huang, M.D. Preparation and Photodynamic activity of novel monosubstituted amphiphilic Zinc phthalocyanine. Chem. J. China Univ. 2008, 29, 2131–2137. https://doi.org/10.3321/j.issn:0251-0790.2008.11.003. (In Chinese).
- 14.Chen, D.; Song, M.R.; Huang, J.L.; Chen, N.S.; Xue, J.P.; Huang, M.D. Photocyanine: A novel and effective phthalocyanine-based photosensitizer for cancer treatment. J. Innov. Opt. Health Sci. 2020, 13, 2030009. https://doi.org/10.1142/S1793545820300098.
- 15.Chen, J.; Hou, L.; Zheng, K.; Wang, J.; Chen, N.; Huang, J.; Wu, M.; Xue, J. Blood distribution and plasma protein binding of PHOTOCYANINE: A promising phthalocyanine photosensitizer inphaseⅡ clinical trials. Eur. J. Pharm. Sci.Off. J. Eur. Fed. Pharm. Sci. 2020, 153, 105491. https://doi.org/10.1016/j.ejps.2020.105491.
- 16.Chen, H.W.; Chen, J.C.; Chen, N.S.; Huang, J.L.; Wang, J.D.; Huang, M.D. Applications of Peptide Conjugated Photosensitizers in Photodynamic Therapy. Prog. Biochem. Biophys. 2010, 36, 1106–1113. https://doi.org/10.3724/sp.j.1206.2009.00080.
- 17.Chen, Z.; Zhou, S.Y.; Chen, J.C.; Li, L.S.; Hu, P.; Chen, S.; Huang, M.D. An effective zinc phthalocyanine derivative for photodynamic antimicrobial chemotherapy. J. Lumin. 2014, 152, 103–107. https://doi.org/10.1016/j.jlumin.2013.10.067.
- 18.Xiang, Y.L.; Tang, D.Y.; Yan, L.L.; Deng, L.L.; Wang, X.H.; Liu, X.Y.; Zhou, Q.H. Poly-l-lysine modified MOF nanoparticles with pH/ROS sensitive CIP release and CUR triggered photodynamic therapy against drug-resistant bacterial infection. Int. J. Biol. Macromol. 2024, 266, 131330. https://doi.org/10.1016/j.ijbiomac.2024.131330.
- 19.Chen, J.C.; Chen, N.S.; Huang, J.F.; Wang, J.D.; Huang, M.D. Derivatizable phthalocyanine with single carboxyl group: Synthesis and purification. Inorg. Chem. Commun. 2006, 9, 313–315.
- 20.Chen, Z.; Zhou, S.; Chen, J.; Deng, Y.; Luo, Z.; Chen, H.; Hamblin, M.R.; Huang, M. Pentalysine β‐Carbonylphthalocyanine Zinc: An Effective Tumor‐Targeting Photosensitizer for Photodynamic Therapy. ChemMedChem 2010, 5, 890–898. https://doi.org/10.1002/cmdc.201000042.
- 21.Li, L.S.; Luo, Z.P.; Chen, Z.; Chen, J.C.; Zhou, S.Y.; Xu, P.; Hu, P.; Wang, J.D.; Chen, N.S.; Huang, J.L.; et al. Enhanced Photodynamic Efficacy of Zinc Phthalocyanine by Conjugating to Heptalysine. Bioconjugate Chem. 2012, 23, 2168–2172.
- 22.Xu, P.; Chen, J.C.; Chen, Z.; Zhou, S.Y.; Hu, P.; Chen, X.Y.; Huang, M.D. Receptor-Targeting Phthalocyanine Photosensitizer for Improving Antitumor Photocytotoxicity. PLoS ONE 2012, 7, e37051. https://doi.org/10.1371/journal.pone.0037051.
- 23.Chen, Z.; Xu, P.; Chen, J.; Chen, H.; Hu, P.; Chen, X.; Lin, L.; Huang, Y.; Zheng, K.; Zhou, S.; et al. Zinc phthalocyanine conjugated with the amino-terminal fragment of urokinase for tumor-targeting photodynamic therapy. Acta Biomater. 2014, 10, 4257–4268. https://doi.org/10.1016/j.actbio.2014.06.026.
- 24.Lin, H.; Chen, J.; Zhang, Y.; Ulla, A.; Liu, J.; Lin, F.; Jiang, L.; Huang, M. Enhanced anti-microbial effect through cationization of a mono-triazatricyclodecane substituted asymmetric phthalocyanine. J. Inorg. Biochem. 2018, 189, 192–198. https://doi.org/10.1016/j.jinorgbio.2018.10.001.
- 25.Liu, D.F.; Li, L.S.; Chen, J.C.; Chen, Z.; Jiang, L.G.; Yuan, C.; Huang, M.D. Dissociation of zinc phthalocyanine aggregation on bacterial surface is key for photodynamic antimicrobial effect. J. Porphyr. Phthalocyanines 2018, 22, 925–934. https://doi.org/10.1142/S1088424618500888.
- 26.Cui, Y.; Wang, H.L.; Yin, F.; Xu, Z.; Qin, X.B.; Yu, K.N.; Cheng, P.P. Safety evaluation of amino acid photosensitive disinfectant. J. Toxicol. 2015, 29, 477–479. https://doi.org/10.16421/j.cnki.1002-3127.2015.06.021.
- 27.Chen, J.; Chen, Z.; Zheng, Y.; Zhou, S.; Wang, J.; Chen, N.; Huang, J.; Yan, F.; Huang, M. Substituted zinc phthalocyanine as an antimicrobial photosensitizer for periodontitis treatment. J. Porphyr. Phthalocyanines 2012, 15, 293–299. https://doi.org/10.1142/s1088424611003276.
- 28.Chen, Z.; Zhang, Y.X.; Wang, D.; Li, L.S.; Zhou, S.Y.; Huang, J.H.; Chen, J.C.; Hu, P.; Huang, M.D. Photodynamic antimicrobial chemotherapy using zinc phthalocyanine derivatives in treatment of bacterial skin infection. J. Biomed. Opt. 2016, 21, 018001. https://doi.org/10.1117/1.Jbo.21.1.018001.
- 29.Wang, D.; Zhang, Y.X.; Yan, S.F.; Chen, Z.H.; Deng, Y.C.; Xu, P.; Chen, J.C.; Liu, W.Z.; Hu, P.; Huang, M.D.; et al. An effective zinc phthalocyanine derivative against multidrug-resistant bacterial infection. J. Porphyr. Phthalocyanines 2017, 21, 205–210. https://doi.org/10.1142/S1088424617500298.
- 30.Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials-are we afraid of the light? Lancet Infect. Dis. 2017, 17, e49-e55. https://doi.org/10.1016/s1473-3099(16)30268-7.
- 31.Bertoloni, G.; Lauro, F.M.; Cortella, G.; Merchat, M. Photosensitizing activity of hematoporphyrin on Staphylococcus aureus cells. Biochim. Biophys. Acta 2000, 1475, 169–174. https://doi.org/10.1016/s0304-4165(00)00071-4.
- 32.Jori, G.; Fabris, C.; Soncin, M.; Ferro, S.; Coppellotti, O.; Dei, D.; Fantetti, L.; Chiti, G.; Roncucci, G. Photodynamic therapy in the treatment of microbial infections: Basic principles and perspective applications. Lasers Surg. Med. 2006, 38, 468–481. https://doi.org/10.1002/lsm.20361.
- 33.Xiling, G.; Yin, C.; Ling, W.; Xiaosong, W.; Jingjing, F.; Fang, L.; Xiaoyan, Z.; Yiyue, G.; Ying, C.; Lunbiao, C.; et al. In vitro inactivation of SARS-CoV-2 by commonly used disinfection products and methods. Sci. Rep. 2021, 11, 2418. https://doi.org/10.1038/s41598-021-82148-w.
- 34.Yu, S.J.; Sun, G.H.; Sui, Y.Q.; Li, H.L.; Mai, Y.H.; Wang, G.D.; Zhang, N.; Bi, Y.H.; Gao, G.F.; Xu, P.; et al. Potent inhibition of Severe Acute Respiratory Syndrome Coronavirus 2 by photosensitizers compounds. Dye. Pigment. 2021, 194, 109570. https://doi.org/10.1016/j.dyepig.2021.109570.
- 35.Li, L.L.; Chen, D.; Zheng, K.; Jiang, L.B.; Dai, T.; Yang, L.; Jiang, L.G.; Chen, Z.; Yuan, C.; Huang, M.D. Enhanced Antitumor Efficacy and Imaging Application of Photosensitizer-Formulated Paclitaxel. ACS Appl. Mater. Interfaces 2020, 12, 4221–4230. https://doi.org/10.1021/acsami.9b18396.
- 36.Chen, D.; Liu, P.W.; Liu, Y.R.; Wang, Z.Y.; Zhou, Y.; Jiang, L.G.; Yuan, C.; Li, Y.K.; Lin, W.; Huang, M.D. A Clot-Homing Near-Infrared Probe for In Vivo Imaging of Murine Thromboembolic Models. Adv. Healthc. Mater. 2022, 11, e2102213. https://doi.org/10.1002/adhm.202102213.
- 37.Jiang, L.B.; Liu, Y.R.; Xu, X.Y.; Su, D.; Zou, H.S.; Liu, J.Y.; Yuan, C.; Huang, M.D. Inhibition of the Citrus Canker Pathogen Using a Photosensitizer Assisted by Sunlight Irradiation. Front. Microbiol. 2020, 11, 571691. https://doi.org/10.3389/fmicb.2020.571691.
- 38.Li, Y.M. Stain removal and whitening by baking soda dentifrice A review of literature. J. Am. Dent. Assoc. 2017, 148, S20–S26. https://doi.org/10.1016/j.adaj.2017.09.006.
- 39.Li, Z.; Wu, Z.; Wang, J.; Huang, M.; Lin, M. Expanding the applications of photodynamic therapy—Tooth bleaching. Clin. Oral Investig. 2021, 26, 2175–2186. https://doi.org/10.1007/s00784-021-04199-7.
- 40.Wu, Z.; Wang, G.; Li, Z.; Li, Z.; Huang, D.; Huang, M.; Lin, M. Dental Bleaching with Phthalocyanine Photosensitizers: Effects on Dentin Color and Collagen Content. Molecules 2023, 28, 4223. https://doi.org/10.3390/molecules28104223.
- 41.Liu, D.; Jiang, L.; Chen, J.; Chen, Z.; Yuan, C.; Lin, D.; Huang, M. Monomer and Oligomer Transition of Zinc Phthalocyanine Is Key for Photobleaching in Photodynamic Therapy. Molecules 2023, 28, 4639. https://doi.org/10.3390/molecules28124639.
- 42.Bunin, D.A.; Martynov, A.G.; Gvozdev, D.A.; Gorbunova, Y.G. Phthalocyanine aggregates in the photodynamic therapy: Dogmas, controversies, and future prospects. Biophys. Rev. 2023, 15, 983–998. https://doi.org/10.1007/s12551-023-01129-7.
- 43.Zhang, Y.; Zheng, K.; Chen, Z.; Chen, J.; Hu, P.; Cai, L.; Iqbal, Z.; Huang, M. Rapid killing of bacteria by a new type of photosensitizer. Appl. Microbiol. Biotechnol. 2017, 101, 4691–4700. https://doi.org/10.1007/s00253-017-8133-8.
- 44.Chinna Ayya Swamy, P.; Sivaraman, G.; Priyanka, R.N.; Raja, S.O.; Ponnuvel, K.; Shanmugpriya, J.; Gulyani, A. Near Infrared (NIR) absorbing dyes as promising photosensitizer for photo dynamic therapy. Coord. Chem. Rev. 2020, 411, 213233. https://doi.org/10.1016/j.ccr.2020.213233.
How to Cite
Chen, Z.; Chen, J.; Liu, D.; Chen, J.; Li, L.; Chen, D.; Chen, N.; Huang, J.; Chen, Z.; Xu, P.; Jiang, L.; Yuan, C.; Jiang, Y.; Huang, M. A Simple Phthalocyanine-Peptide Conjugate as Targeting Photosensitizer and Its Broad Applications in Health. Health and Metabolism 2024, 1 (1), 3. https://doi.org/10.53941/hm.2024.100003.
RIS
BibTex
Copyright & License

Copyright (c) 2024 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


