Satellite cells, the resident muscle stem cells, play a crucial role in skeletal muscle regeneration, growth, and repair. Asymmetric cell division is a critical process regulating satellite cell self-renewal and differentiation, and is governed by various intrinsic and extrinsic factors. Key biomarkers of satellite cell characteristics, such as Pax7, MRFs, and Sprouty1, are essential in maintaining satellite cell homeostasis. Signaling pathways, including Notch, Wnt, TGF-β, FGF2, and the PAR complex, intricately regulate satellite cell division and fate determination. Asymmetric division is orchestrated through the establishment of cell polarity and differential distribution of fate determinants. Aging and diseases like Duchenne muscular dystrophy disrupt asymmetric division, leading to impaired satellite cell function and muscle regeneration. Potential therapeutic strategies aim to rejuvenate satellite cells and promote muscle regeneration by targeting the gut microbiome, utilizing gene editing technologies, and harnessing the power of exercise-induced factors. Understanding the molecular mechanisms governing satellite cell behavior and Keywords should be in lowercase and separated by semicolons. Developing innovative therapies hold promise for combating age-related muscle deterioration and pathological conditions characterized due to impaired muscle regeneration. Future research should focus on unraveling the complex regulatory networks and translating findings into effective clinical applications to restore muscle function.
- Open Access
- Review
Asymmetric Cell Division and Satellite Cell Fate Regulation in Skeletal Muscle Aging and Disease
- Shenghe Wang 1, 2,
- Guangchuang Yu 3,
- Liwei Xie 1, 4, 5, *
Author Information
Received: 29 Jul 2024 | Revised: 30 Sep 2024 | Accepted: 20 Oct 2024 | Published: 30 Oct 2024
Abstract
Graphical Abstract
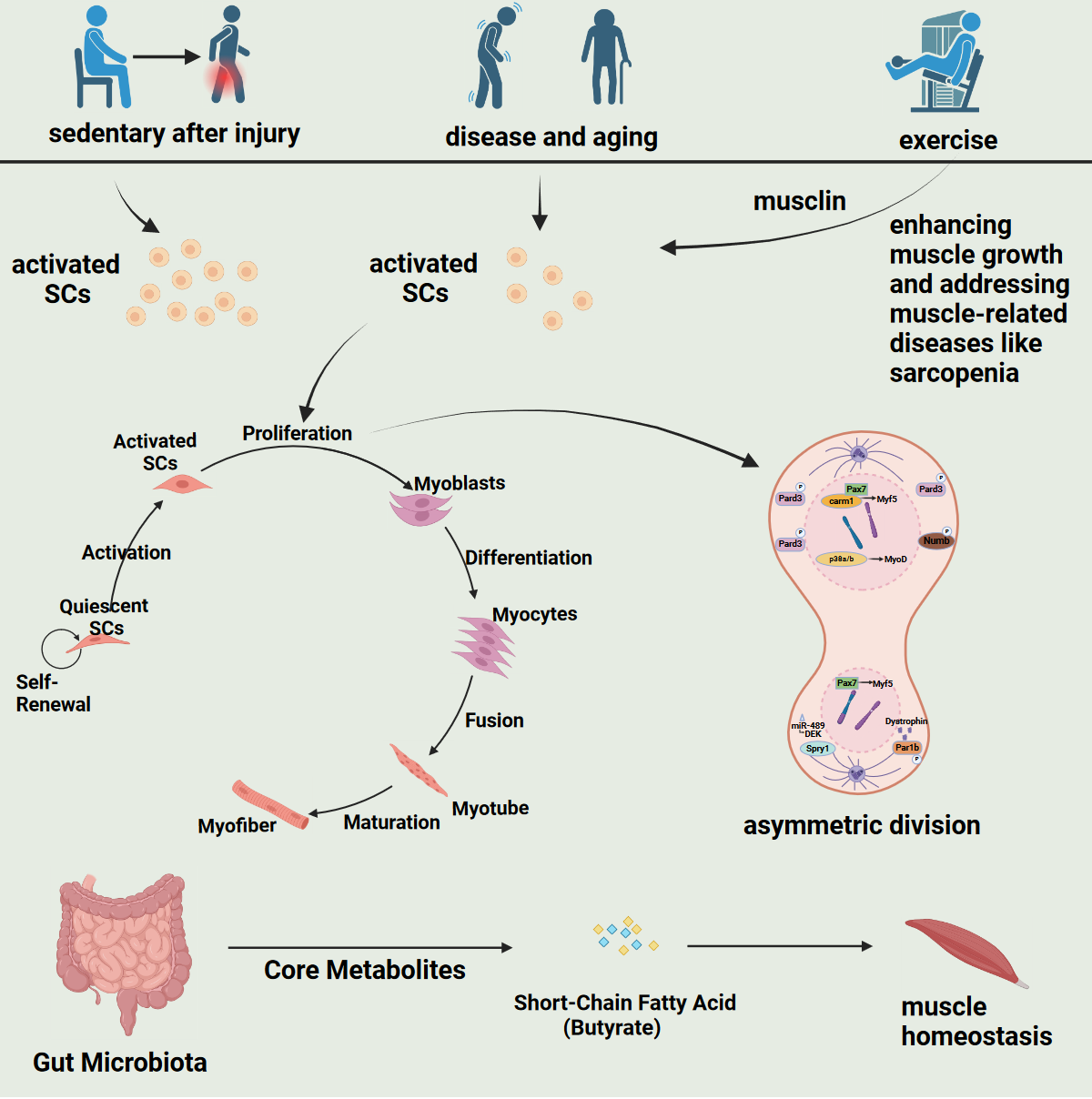
References
- 1.Relaix, F.; Bencze, M.; Borok, M.J.; Der Vartanian, A.; Gattazzo, F.; Mademtzoglou, D.; Perez-Diaz, S.; Prola, A.; Reyes-Fernandez, P.C.; Rotini, A.; et al. Perspectives on Skeletal Muscle Stem Cells. Nat. Commun. 2021, 12, 692. https://doi.org/10.1038/s41467-020-20760-6.
- 2.Fu, X.; Zhuang, C.; Hu, P. Regulation of Muscle Stem Cell Fate. Cell Regen. 2022, 11, 40. https://doi.org/10.1186/s13619-022-00142-7.
- 3.Sousa-Victor, P.; García-Prat, L.; Muñoz-Cánoves, P. Control of Satellite Cell Function in Muscle Regeneration and Its Disruption in Ageing. Nat. Rev. Mol. Cell Biol. 2022, 23, 204–226. https://doi.org/10.1038/s41580-021-00421-2.
- 4.Peng, J.; Han, L.; Liu, B.; Song, J.; Wang, Y.; Wang, K.; Guo, Q.; Liu, X.; Li, Y.; Zhang, J.; et al. Gli1 Marks a Sentinel Muscle Stem Cell Population for Muscle Regeneration. Nat. Commun. 2023, 14, 6993. https://doi.org/10.1038/s41467-023-42837-8.
- 5.AMPKα2 Is a Skeletal Muscle Stem Cell Intrinsic Regulator of Myonuclear Accretion–PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/38077152/ (accessed on 19 March 2024).
- 6.Hong, X.; Isern, J.; Campanario, S.; Perdiguero, E.; Ramírez-Pardo, I.; Segalés, J.; Hernansanz-Agustín, P.; Curtabbi, A.; Deryagin, O.; Pollán, A.; et al. Mitochondrial Dynamics Maintain Muscle Stem Cell Regenerative Competence throughout Adult Life by Regulating Metabolism and Mitophagy. Cell Stem Cell 2022, 29, 1298–1314.e10. https://doi.org/10.1016/j.stem.2022.07.009.
- 7.Sastourné-Arrey, Q.; Mathieu, M.; Contreras, X.; Monferran, S.; Bourlier, V.; Gil-Ortega, M.; Murphy, E.; Laurens, C.; Varin, A.; Guissard, C.; et al. Adipose Tissue Is a Source of Regenerative Cells That Augment the Repair of Skeletal Muscle after Injury. Nat. Commun. 2023, 14, 80. https://doi.org/10.1038/s41467-022-35524-7.
- 8.Wosczyna, M.N.; Konishi, C.T.; Perez Carbajal, E.E.; Wang, T.T.; Walsh, R.A.; Gan, Q.; Wagner, M.W.; Rando, T.A. Mesenchymal Stromal Cells Are Required for Regeneration and Homeostatic Maintenance of Skeletal Muscle. Cell Rep. 2019, 27, 2029–2035.e5. https://doi.org/10.1016/j.celrep.2019.04.074.
- 9.Lukjanenko, L.; Karaz, S.; Stuelsatz, P.; Gurriaran-Rodriguez, U.; Michaud, J.; Dammone, G.; Sizzano, F.; Mashinchian, O.; Ancel, S.; Migliavacca, E.; et al. Aging Disrupts Muscle Stem Cell Function by Impairing Matricellular WISP1 Secretion from Fibro-Adipogenic Progenitors. Cell Stem Cell 2019, 24, 433–446.e7. https://doi.org/10.1016/j.stem.2018.12.014.
- 10.Shang, M.; Cappellesso, F.; Amorim, R.; Serneels, J.; Virga, F.; Eelen, G.; Mazzone, M. Macrophage-derived glutamine boosts satellite cells and muscle regeneration. Nature 2020, 587, 626–631.
- 11.Zhang, C.; Cheng, N.; Qiao, B.; Zhang, F.; Wu, J.; Liu, C.; Li, Y.; Du, J. Age-related Decline of Interferon-gamma Responses in Macrophage Impairs Satellite Cell Proliferation and Regeneration. J. Cachexia Sarcopenia Muscle 2020, 11, 1291–1305. https://doi.org/10.1002/jcsm.12584.
- 12.Southerland, K.W.; Xu, Y.; Peters, D.T.; Lin, X.; Wei, X.; Xiang, Y.; Fei, K.; Olivere, L.A.; Morowitz, J.M.; Otto, J.; et al. Skeletal Muscle Regeneration Failure in Ischemic-Damaged Limbs Is Associated with pro-Inflammatory Macrophages and Premature Differentiation of Satellite Cells. Genome Med. 2023, 15, 95. https://doi.org/10.1186/s13073-023-01250-y.
- 13.Burzyn, D.; Kuswanto, W.; Kolodin, D.; Shadrach, J.L.; Cerletti, M.; Jang, Y.; Sefik, E.; Tan, T.G.; Wagers, A.J.; Benoist, C.; et al. A Special Population of Regulatory T Cells Potentiates Muscle Repair. Cell 2013, 155, 1282–1295. https://doi.org/10.1016/j.cell.2013.10.054.
- 14.Becker, M.; Joseph, S.S.; Garcia-Carrizo, F.; Tom, R.Z.; Opaleva, D.; Serr, I.; Tschöp, M.H.; Schulz, T.J.; Hofmann, S.M.; Daniel, C. Regulatory T Cells Require IL6 Receptor Alpha Signaling to Control Skeletal Muscle Function and Regeneration. Cell Metab. 2023, 35, 1736–1751.e7. https://doi.org/10.1016/j.cmet.2023.08.010.
- 15.Seale, P.; Sabourin, L.A.; Girgis-Gabardo, A.; Mansouri, A.; Gruss, P.; Rudnicki, M.A. Pax7 Is Required for the Specification of Myogenic Satellite Cells. Cell 2000, 102, 777–786. https://doi.org/10.1016/S0092-8674(00)00066-0.
- 16.Sambasivan, R.; Yao, R.; Kissenpfennig, A.; Van Wittenberghe, L.; Paldi, A.; Gayraud-Morel, B.; Guenou, H.; Malissen, B.; Tajbakhsh, S.; Galy, A. Pax7-Expressing Satellite Cells Are Indispensable for Adult Skeletal Muscle Regeneration. Development 2011, 138, 4333–4333. https://doi.org/10.1242/dev.073601.
- 17.Saber, J.; Rudnicki, M.A. Carm1 and the Epigenetic Control of Stem Cell Function. Stem Cells Transl. Med. 2022, 11, 1143–1150. https://doi.org/10.1093/stcltm/szac068.
- 18.Davis, R.L.; Weintraub, H.; Lassar, A.B. Expression of a Single Transfected cDNA Converts Fibmblasts to Myoblasts. Cell 1987, 51, 987–1000.
- 19.Weintraub, H.; Tapscott, S.J.; Davis, R.L.; Thayer, M.J.; Adam, M.A.; Lassar, A.B.; Miller, A.D. Activation of Muscle-Specific Genes in Pigment, Nerve, Fat, Liver, and Fibroblast Cell Lines by Forced Expression of MyoD. Proc. Natl. Acad. Sci. USA 1989, 86, 5434–5438. https://doi.org/10.1073/pnas.86.14.5434.
- 20.Megeney, L.A.; Kablar, B.; Garrett, K.; Anderson, J.E.; Rudnicki, M.A. MyoD Is Required for Myogenic Stem Cell Function in Adult Skeletal Muscle. Genes. Dev. 1996, 10, 1173–1183. https://doi.org/10.1101/gad.10.10.1173.
- 21.Sabourin, L.A.; Girgis-Gabardo, A.; Seale, P.; Asakura, A.; Rudnicki, M.A. Reduced Differentiation Potential of Primary MyoD−/− Myogenic Cells Derived from Adult Skeletal Muscle. J. Cell Biol. 1999, 144, 631–643.
- 22.Chen, Y.-H.; Wang, Y.-H.; Chang, M.-Y.; Lin, C.-Y.; Weng, C.-W.; Westerfield, M.; Tsai, H.-J. Multiple Upstream Modules Regulate Zebrafish Myf5expression. BMC Dev. Biol. 2007, 7, 1. https://doi.org/10.1186/1471-213X-7-1.
- 23.Zhang, P.; Li, W.; Wang, L.; Liu, H.; Gong, J.; Wang, F.; Chen, X. Salidroside Inhibits Myogenesis by Modulating P-Smad3-Induced Myf5 Transcription. Front. Pharmacol. 2018, 9, 209. https://doi.org/10.3389/fphar.2018.00209.
- 24.Sato, T.; Rocancourt, D.; Marques, L.; Thorsteinsdóttir, S.; Buckingham, M. A Pax3/Dmrt2/Myf5 Regulatory Cascade Functions at the Onset of Myogenesis. PLoS Genet. 2010, 6, e1000897. https://doi.org/10.1371/journal.pgen.1000897.
- 25.Li, Q.; Zhu, X.; Yu, C.; Shang, L.; Li, R.; Wang, X.; Yang, Y.; Meng, J.; Kong, X. Case Report: A Novel Homozygous Mutation in MYF5 Due to Paternal Uniparental Isodisomy of Chromosome 12 in a Case of External Ophthalmoplegia With Rib and Vertebral Anomalies. Front. Genet. 2022, 12, 780363. https://doi.org/10.3389/fgene.2021.780363.
- 26.Doucet, C.; Gutierrez, G.J.; Lindon, C.; Lorca, T.; Lledo, G.; Pinset, C.; Coux, O. Multiple Phosphorylation Events Control Mitotic Degradation of the Muscle Transcription Factor Myf5. BMC Biochem. 2005, 6, 27. https://doi.org/10.1186/1471-2091-6-27.
- 27.Hadchouel, J.; Tajbakhsh, S.; Primig, M.; Chang, T.H.-T.; Daubas, P.; Rocancourt, D.; Buckingham, M. Modular Long-Range Regulation of Myf5 Reveals Unexpected Heterogeneity between Skeletal Muscles in the Mouse Embryo. Development 2000, 127, 4455–4467. https://doi.org/10.1242/dev.127.20.4455.
- 28.Ganassi, M.; Badodi, S.; Wanders, K.; Zammit, P.S.; Hughes, S.M. Myogenin Is an Essential Regulator of Adult Myofibre Growth and Muscle Stem Cell Homeostasis. eLife 2020, 9, e60445. https://doi.org/10.7554/eLife.60445.
- 29.Benavente-Diaz, M.; Comai, G.; Di Girolamo, D.; Langa, F.; Tajbakhsh, S. Dynamics of Myogenic Differentiation Using a Novel Myogenin Knock-in Reporter Mouse. Skelet. Muscle 2021, 11, 5. https://doi.org/10.1186/s13395-021-00260-x.
- 30.Lazure, F.; Blackburn, D.M.; Corchado, A.H.; Sahinyan, K.; Karam, N.; Sharanek, A.; Nguyen, D.; Lepper, C.; Najafabadi, H.S.; Perkins, T.J.; et al. Myf6/MRF4 Is a Myogenic Niche Regulator Required for the Maintenance of the Muscle Stem Cell Pool. EMBO Rep. 2020, 21, e49499. https://doi.org/10.15252/embr.201949499.
- 31.Shea, K.L.; Xiang, W.; LaPorta, V.S.; Licht, J.D.; Keller, C.; Basson, M.A.; Brack, A.S. Sprouty1 Regulates Reversible Quiescence of a Self-Renewing Adult Muscle Stem Cell Pool during Regeneration. Cell Stem Cell 2010, 6, 117–129. https://doi.org/10.1016/j.stem.2009.12.015.
- 32.Chakkalakal, J.V.; Jones, K.M.; Basson, M.A.; Brack, A.S. The Aged Niche Disrupts Muscle Stem Cell Quiescence. Nature 2012, 490, 355–360. https://doi.org/10.1038/nature11438.
- 33.Bigot, A.; Duddy, W.J.; Ouandaogo, Z.G.; Negroni, E.; Mariot, V.; Ghimbovschi, S.; Harmon, B.; Wielgosik, A.; Loiseau, C.; Devaney, J.; et al. Age-Associated Methylation Suppresses SPRY1, Leading to a Failure of Re-Quiescence and Loss of the Reserve Stem Cell Pool in Elderly Muscle. Cell Rep. 2015, 13, 1172–1182. https://doi.org/10.1016/j.celrep.2015.09.067.
- 34.Xie, L.; Yin, A.; Nichenko, A.S.; Beedle, A.M.; Call, J.A.; Yin, H. Transient HIF2A Inhibition Promotes Satellite Cell Proliferation and Muscle Regeneration. J. Clin. Investig. 2018, 128, 2339–2355. https://doi.org/10.1172/JCI96208.
- 35.Meng, J.; Lv, Z.; Chen, X.; Sun, C.; Jin, C.; Ding, K.; Chen, C. LBP1C-2 from Lycium Barbarum Maintains Skeletal Muscle Satellite Cell Pool by Interaction with FGFR1. iScience 2023, 26, 106573. https://doi.org/10.1016/j.isci.2023.106573.
- 36.Dumont, N.A.; Wang, Y.X.; Von Maltzahn, J.; Pasut, A.; Bentzinger, C.F.; Brun, C.E.; Rudnicki, M.A. Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nat. Med. 2015, 21, 1455–1463.
- 37.Conboy, I.M.; Rando, T.A. The Regulation of Notch Signaling Controls Satellite Cell Activation and Cell Fate Determination in Postnatal Myogenesis. Developmental Cell 2002, 3, 397–409.
- 38.Conboy, I.M.; Conboy, M.J.; Wagers, A.J.; Girma, E.R.; Weissman, I.L.; Rando, T.A. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005, 433, 760–764.
- 39.Servián-Morilla, E.; Takeuchi, H.; Lee, T.V.; Clarimon, J.; Mavillard, F.; Area-Gómez, E.; Rivas, E.; Nieto-González, J.L.; Rivero, M.C.; Cabrera, M.; et al. A POGLUT1 Mutation Causes a Muscular Dystrophy with Reduced Notch Signaling and Satellite Cell Loss. EMBO Mol. Med. 2016, 8, 1289–1309.
- 40.Baghdadi, M.B.; Firmino, J.; Soni, K.; Evano, B.; Di Girolamo, D.; Mourikis, P.; Tajbakhsh, S. Notch-induced miR-708 antagonizes satellite cell migration and maintains quiescence. Cell Stem Cell 2018, 23, 859–868. e5.
- 41.Wei, X.; Rigopoulos, A.; Lienhard, M.; Pöhle-Kronawitter, S.; Kotsaris, G.; Franke, J.; Stricker, S. Neurofibromin 1 controls metabolic balance and Notch-dependent quiescence of murine juvenile myogenic progenitors. Nat. Commun. 2024, 15, 1393.
- 42.Brack, A.S.; Conboy, M.J.; Roy, S.; Lee, M.; Kuo, C.J.; Keller, C.; Rando, T.A. Increased Wnt Signaling during Aging Alters Muscle Stem Cell Fate and Increases Fibrosis. Science 2007, 317, 807–810. https://doi.org/10.1126/science.1144090.
- 43.Brack, A.S.; Conboy, I.M.; Conboy, M.J.; Shen, J.; Rando, T.A. A Temporal Switch from Notch to Wnt Signaling in Muscle Stem Cells Is Necessary for Normal Adult Myogenesis. Cell Stem Cell 2008, 2, 50–59. https://doi.org/10.1016/j.stem.2007.10.006.
- 44.Le Grand, F.; Jones, A.E.; Seale, V.; Scimè, A.; Rudnicki, M.A. Wnt7a Activates the Planar Cell Polarity Pathway to Drive the Symmetric Expansion of Satellite Stem Cells. Cell Stem Cell 2009, 4, 535–547. https://doi.org/10.1016/j.stem.2009.03.013.
- 45.Carlson, M.E.; Hsu, M.; Conboy, I.M. Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells. Nature 2008, 454, 528–532.
- 46.Ge, X.; McFarlane, C.; Vajjala, A.; Lokireddy, S.; Ng, Z.H.; Tan, C.K.; Kambadur, R. Smad3 signaling is required for satellite cell function and myogenic differentiation of myoblasts. Cell Res. 2011, 21, 1591–1604.
- 47.Teng, H.; Zheng, J.; Liang, Y.; Zhao, J.; Yan, Y.; Li, S.; Tong, H. Podocan promoting skeletal muscle post‐injury regeneration by inhibiting TGF‐β signaling pathway. FASEB J. 2024, 38, e23502.
- 48.Rozo, M.; Li, L.; Fan, C.-M. Targeting Β1-Integrin Signaling Enhances Regeneration in Aged and Dystrophic Muscle in Mice. Nat. Med. 2016, 22, 889–896.
- 49.Feige, P.; Brun, C.E.; Ritso, M.; Rudnicki, M.A. Orienting muscle stem cells for regeneration in homeostasis, aging, and disease. Cell Stem Cell 2018, 23, 653–664.
- 50.Troy, A.; Cadwallader, A.B.; Fedorov, Y.; Tyner, K.; Tanaka, K.K.; Olwin, B.B. Coordination of Satellite Cell Activation and Self-Renewal by Par-Complex-Dependent Asymmetric Activation of P38α/β MAPK. Cell Stem Cell 2012, 11, 541–553. https://doi.org/10.1016/j.stem.2012.05.025.
- 51.Bernet, J.D.; Doles, J.D.; Hall, J.K.; Kelly Tanaka, K.; Carter, T.A.; Olwin, B.B. P38 MAPK Signaling Underlies a Cell-Autonomous Loss of Stem Cell Self-Renewal in Skeletal Muscle of Aged Mice. Nat. Med. 2014, 20, 265–271. https://doi.org/10.1038/nm.3465.
- 52.Reano, S.; Angelino, E.; Ferrara, M.; Malacarne, V.; Sustova, H.; Sabry, O.; Filigheddu, N. Unacylated ghrelin enhances satellite cell function and relieves the dystrophic phenotype in duchenne muscular dystrophy mdx model. Stem Cells 2017, 35, 1733–1746.
- 53.Massenet, J.; Gardner, E.; Chazaud, B.; Dilworth, F.J. Epigenetic Regulation of Satellite Cell Fate during Skeletal Muscle Regeneration. Skeletal Muscle 2021, 11, 4. https://doi.org/10.1186/s13395-020-00259-w.
- 54.Liu, L.; Cheung, T.H.; Charville, G.W.; Hurgo, B.M.C.; Leavitt, T.; Shih, J.; Brunet, A.; Rando, T.A. Chromatin Modifications as Determinants of Muscle Stem Cell Quiescence and Chronological Aging. Cell Rep. 2013, 4, 189–204. https://doi.org/10.1016/j.celrep.2013.05.043.
- 55.Naito, M.; Mori, M.; Inagawa, M.; Miyata, K.; Hashimoto, N.; Tanaka, S.; Asahara, H. Dnmt3a Regulates Proliferation of Muscle Satellite Cells via p57Kip2. PLoS Genet. 2016, 12, e1006167. https://doi.org/10.1371/journal.pgen.1006167.
- 56.Marroncelli, N.; Bianchi, M.; Bertin, M.; Consalvi, S.; Saccone, V.; De Bardi, M.; Puri, P.L.; Palacios, D.; Adamo, S.; Moresi, V. HDAC4 Regulates Satellite Cell Proliferation and Differentiation by Targeting P21 and Sharp1 Genes. Sci. Rep. 2018, 8, 3448. https://doi.org/10.1038/s41598-018-21835-7.
- 57.Zhang, N.; Mendieta-Esteban, J.; Magli, A.; Lilja, K.C.; Perlingeiro, R.C.R.; Marti-Renom, M.A.; Tsirigos, A.; Dynlacht, B.D. Muscle Progenitor Specification and Myogenic Differentiation Are Associated with Changes in Chromatin Topology. Nat. Commun. 2020, 11, 6222. https://doi.org/10.1038/s41467-020-19999-w.
- 58.Liu, N.; Williams, A.H.; Kim, Y.; McAnally, J.; Bezprozvannaya, S.; Sutherland, L.B.; Olson, E.N. An intragenic MEF2-dependent enhancer directs muscle-specific expression of microRNAs 1 and 133. Proc. Natl. Acad. Sci. USA 2007, 104, 20844–20849.
- 59.Liu, N.; Bezprozvannaya, S.; Shelton, J.M.; Frisard, M.I.; Hulver, M.W.; McMillan, R.P.; Olson, E.N. Mice lacking microRNA 133a develop dynamin 2–dependent centronuclear myopathy. J. Clin. Investig. 2011, 121, 3258–3268.
- 60.Horak, M.; Novak, J.; Bienertova-Vasku, J. Muscle-Specific microRNAs in Skeletal Muscle Development. Dev. Biol. 2016, 410, 1–13. https://doi.org/10.1016/j.ydbio.2015.12.013.
- 61.Kuang, S.; Kuroda, K.; Le Grand, F.; Rudnicki, M.A. Asymmetric Self-Renewal and Commitment of Satellite Stem Cells in Muscle. Cell 2007, 129, 999–1010. https://doi.org/10.1016/j.cell.2007.03.044.
- 62.Chang, N.C.; Sincennes, M.-C.; Chevalier, F.P.; Brun, C.E.; Lacaria, M.; Segalés, J.; Muñoz-Cánoves, P.; Ming, H.; Rudnicki, M.A. The Dystrophin Glycoprotein Complex Regulates the Epigenetic Activation of Muscle Stem Cell Commitment. Cell Stem Cell 2018, 22, 755–768.e6. https://doi.org/10.1016/j.stem.2018.03.022.
- 63.Dumont, N.A.; Rudnicki, M.A. Targeting Muscle Stem Cell Intrinsic Defects to Treat Duchenne Muscular Dystrophy. NPJ Regen. Med. 2016, 1, 16006–. https://doi.org/10.1038/npjregenmed.2016.6.
- 64.Wang, Y.X.; Feige, P.; Brun, C.E.; Hekmatnejad, B.; Dumont, N.A.; Renaud, J.-M.; Faulkes, S.; Guindon, D.E.; Rudnicki, M.A. EGFR-Aurka Signaling Rescues Polarity and Regeneration Defects in Dystrophin-Deficient Muscle Stem Cells by Increasing Asymmetric Divisions. Cell Stem Cell 2019, 24, 419–432.e6. https://doi.org/10.1016/j.stem.2019.01.002.
- 65.Kim, K.M.; Yoo, G.D.; Heo, W.; Oh, H.T.; Park, J.; Shin, S.; Do, Y.; Jeong, M.G.; Hwang, E.S.; Hong, J. TAZ Stimulates Exercise-induced Muscle Satellite Cell Activation via Pard3–P38 MAPK–TAZ Signalling Axis. J. Cachexia Sarcopenia Muscle 2023, 14, 2733–2746. https://doi.org/10.1002/jcsm.13348.
- 66.Dewey, E.; Taylor, D.; Johnston, C. Cell Fate Decision Making through Oriented Cell Division. JDB 2015, 3, 129–157. https://doi.org/10.3390/jdb3040129.
- 67.Sunchu, B.; Cabernard, C. Principles and mechanisms of asymmetric cell division. Development 2020, 147, dev167650.
- 68.Shinin, V.; Gayraud-Morel, B.; Gomès, D.; Tajbakhsh, S. Asymmetric Division and Cosegregation of Template DNA Strands in Adult Muscle Satellite Cells. Nat. Cell Biol. 2006, 8, 677–682. https://doi.org/10.1038/ncb1425.
- 69.Rocheteau, P.; Gayraud-Morel, B.; Siegl-Cachedenier, I.; Blasco, M.A.; Tajbakhsh, S. A Subpopulation of Adult Skeletal Muscle Stem Cells Retains All Template DNA Strands after Cell Division. Cell 2012, 148, 112–125. https://doi.org/10.1016/j.cell.2011.11.049.
- 70.Cheung, T.H.; Quach, N.L.; Charville, G.W.; Liu, L.; Park, L.; Edalati, A.; Yoo, B.; Hoang, P.; Rando, T.A. Maintenance of Muscle Stem-Cell Quiescence by microRNA-489. Nature 2012, 482, 524–528. https://doi.org/10.1038/nature10834.
- 71.Welle, S. Cellular and Molecular Basis of Age-Related Sarcopenia. Can. J. Appl. Physiol. 2002, 27. https://doi.org/10.1139/h02-002.
- 72.Jejurikar, S.S.; Henkelman, E.A.; Cederna, P.S.; Marcelo, C.L.; Urbanchek, M.G.; Kuzon, W.M. Aging Increases the Susceptibility of Skeletal Muscle Derived Satellite Cells to Apoptosis. Exp. Gerontol. 2006, 41, 828–836. https://doi.org/10.1016/j.exger.2006.06.053.
- 73.Hwang, A.B.; Brack, A.S. Muscle Stem Cells and Aging. Curr. Top. Dev. Biol. 2018, 126, 299–322. https://doi.org/10.1016/bs.ctdb.2017.08.008.
- 74.Cosgrove, B.D.; Gilbert, P.M.; Porpiglia, E.; Mourkioti, F.; Lee, S.P.; Corbel, S.Y.; Llewellyn, M.E.; Delp, S.L.; Blau, H.M. Rejuvenation of the Muscle Stem Cell Population Restores Strength to Injured Aged Muscles. Nat. Med. 2014, 20, 255–264. https://doi.org/10.1038/nm.3464.
- 75.Benjamin, D.I.; Brett, J.O.; Both, P.; Benjamin, J.S.; Ishak, H.L.; Kang, J.; Kim, S.; Chung, M.; Arjona, M.; Nutter, C.W.; et al. Multiomics Reveals Glutathione Metabolism as a Driver of Bimodality during Stem Cell Aging. Cell Metabolism 2023, 35, 472–486.e6. https://doi.org/10.1016/j.cmet.2023.02.001.
- 76.Forcina, L.; Musarò, A. Rejuvenating muscle stem cells with the glutathione system. Cell Metab. 2023, 35, 379–381.
- 77.Cardone, N.; Taglietti, V.; Baratto, S.; Kefi, K.; Periou, B.; Gitiaux, C.; Barnerias, C.; Lafuste, P.; Pharm, F.L.; Pharm, J.N.; et al. Myopathologic Trajectory in Duchenne Muscular Dystrophy (DMD) Reveals Lack of Regeneration Due to Senescence in Satellite Cells. Acta Neuropathol. Commun. 2023, 11, 167. https://doi.org/10.1186/s40478-023-01657-z.
- 78.Tichy, E.D.; Sidibe, D.K.; Tierney, M.T.; Stec, M.J.; Sharifi-Sanjani, M.; Hosalkar, H.; Mubarak, S.; Johnson, F.B.; Sacco, A.; Mourkioti, F. Single Stem Cell Imaging and Analysis Reveals Telomere Length Differences in Diseased Human and Mouse Skeletal Muscles. Stem Cell Rep. 2017, 9, 1328–1341. https://doi.org/10.1016/j.stemcr.2017.08.003.
- 79.Sandonà; M; Esposito, F.; Cargnoni, A.; Silini, A.; Romele, P.; Parolini, O.; Saccone, V. Amniotic membrane-derived stromal cells release extracellular vesicles that favor regeneration of dystrophic skeletal muscles. Int. J. Mol. Sci. 2023, 24, 12457.
- 80.Su, Y.; Cao, Y.; Liu, C.; Xu, Q.; Li, N.; Lan, M.; Li, L.; Wang, K.; Zhang, Z.; Meng, Q. Inactivating IL34 Promotes Regenerating Muscle Stem Cell Expansion and Attenuates Duchenne Muscular Dystrophy in Mouse Models. Theranostics 2023, 13, 2588–2604. https://doi.org/10.7150/thno.83817.
- 81.Taglietti, V.; Kefi, K.; Rivera, L.; Bergiers, O.; Cardone, N.; Coulpier, F.; Gioftsidi, S.; Drayton-Libotte, B.; Hou, C.; Authier, F.-J.; et al. Thyroid-Stimulating Hormone Receptor Signaling Restores Skeletal Muscle Stem Cell Regeneration in Rats with Muscular Dystrophy. Sci. Transl. Med. 2023, 15, eadd5275. https://doi.org/10.1126/scitranslmed.add5275.
- 82.Nance, M.E.; Shi, R.; Hakim, C.H.; Wasala, N.B.; Yue, Y.; Pan, X.; Zhang, T.; Robinson, C.A.; Duan, S.X.; Yao, G.; et al. AAV9 Edits Muscle Stem Cells in Normal and Dystrophic Adult Mice. Molecular Therapy 2019, 27, 1568–1585. https://doi.org/10.1016/j.ymthe.2019.06.012.
- 83.Chen, S.; Zhang, P.; Duan, H.; Wang, J.; Qiu, Y.; Cui, Z.; Xie, L. Gut microbiota in muscular atrophy development, progression and treatment: New therapeutic targets and opportunities. Innovation 2023, 4, 100479.
- 84.Lahiri, S.; Kim, H.; Garcia-Perez, I.; Reza, M.M.; Martin, K.A.; Kundu, P.; Cox, L.M.; Selkrig, J.; Posma, J.M.; Zhang, H.; et al. The Gut Microbiota Influences Skeletal Muscle Mass and Function in Mice. Sci. Transl. Med. 2019, 11, eaan5662. https://doi.org/10.1126/scitranslmed.aan5662.
- 85.Chen, S.; Huang, L.; Liu, B.; Duan, H.; Li, Z.; Liu, Y.; Li, H.; Fu, X.; Lin, J.; Xu, Y.; et al. Dynamic Changes in Butyrate Levels Regulate Satellite Cell Homeostasis by Preventing Spontaneous Activation during Aging. Sci. China Life Sci. 2023. https://doi.org/10.1007/s11427-023-2400-3.
- 86.Hanna, B.S.; Wang, G.; Galván-Peña, S.; Mann, A.O.; Ramirez, R.N.; Muñoz-Rojas, A.R.; Smith, K.; Wan, M.; Benoist, C.; Mathis, D. The Gut Microbiota Promotes Distal Tissue Regeneration via RORγ+ Regulatory T Cell Emissaries. Immunity 2023, 56, 829–846.e8. https://doi.org/10.1016/j.immuni.2023.01.033.
- 87.Jollet, M.; Mariadassou, M.; Rué, O.; Pessemesse, L.; Ollendorff, V.; Ramdani, S.; Vernus, B.; Bonnieu, A.; Bertrand-Gaday, C.; Goustard, B.; et al. Insight into the Role of Gut Microbiota in Duchenne Muscular Dystrophy. Am. J. Pathol. 2024, 194, 264–279. https://doi.org/10.1016/j.ajpath.2023.10.010.
- 88.Kalkan, H.; Pagano, E.; Paris, D.; Panza, E.; Cuozzo, M.; Moriello, C.; Piscitelli, F.; Abolghasemi, A.; Gazzerro, E.; Silvestri, C.; et al. Targeting Gut Dysbiosis against Inflammation and Impaired Autophagy in Duchenne Muscular Dystrophy. EMBO Mol. Med. 2023, 15, e16225. https://doi.org/10.15252/emmm.202216225.
- 89.Guo, Q.; Luo, Q.; Song, G. Control of Muscle Satellite Cell Function by Specific Exercise-induced Cytokines and Their Applications in Muscle Maintenance. J. Cachexia Sarcopenia Muscle 2024, 15, 466–476. https://doi.org/10.1002/jcsm.13440.
- 90.Kang, X.; Qian, J.; Shi, Y.X.; Bian, X.T.; Zhang, L.D.; Li, G.M.; Miao, H.M. Exercise-induced Musclin determines the fate of fibro-adipogenic progenitors to control muscle homeostasis. Cell Stem Cell 2024, 31, 212–226.e7.
- 91.Qaisar, R.; Bhaskaran, S.; Van Remmen, H. Muscle Fiber Type Diversification during Exercise and Regeneration. Free. Radic. Biol. Med. 2016, 98, 56–67. https://doi.org/10.1016/j.freeradbiomed.2016.03.025.
- 92.Distefano, G.; Goodpaster, B.H. Effects of Exercise and Aging on Skeletal Muscle. Cold Spring Harb. Perspect. Med. 2018, 8, a029785. https://doi.org/10.1101/cshperspect.a029785.
- 93.McKendry, J.; Stokes, T.; Mcleod, J.C.; Phillips, S.M. Resistance Exercise, Aging, Disuse, and Muscle Protein Metabolism. In Comprehensive Physiology; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2021; pp. 2249–2278. ISBN 978-0-470-65071-4.
- 94.Hurst, C.; Robinson, S.M.; Witham, M.D.; Dodds, R.M.; Granic, A.; Buckland, C.; De Biase, S.; Finnegan, S.; Rochester, L.; Skelton, D.A.; et al. Resistance Exercise as a Treatment for Sarcopenia: Prescription and Delivery. Age Ageing 2022, 51, afac003. https://doi.org/10.1093/ageing/afac003.
How to Cite
Wang, S.; Yu, G.; Xie, L. Asymmetric Cell Division and Satellite Cell Fate Regulation in Skeletal Muscle Aging and Disease. Health and Metabolism 2024, 1 (1), 5. https://doi.org/10.53941/hm.2024.100005.
RIS
BibTex
Copyright & License

Copyright (c) 2024 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


