The highly pathogenic avian influenza virus H5N1 has garnered global attention due to its high pathogenicity and mortality rates. In recent years, the outbreak of the H5N1 subtype 2.3.4.4b in various mammals has raised concerns about its pandemic potential. This paper reviews the global prevalence and impact of H5N1 virus, explores the current status and challenges in the existing technological platforms for H5N1 vaccine and diagnostic development, and further evaluates the effectiveness and application prospects of current H5N1 therapeutics. This article aims to provide a robust reference to guide the global preparedness for future pandemic potential of avian influenza H5N1.
- Open Access
- Review
H5N1 Avian Influenza: Global Circulation and Response Strategies
- Dongmei Wei 1,
- Xiaoya Wu 1,
- Heming Chen 1,
- Kaiyun Chen 1,
- Ningshao Xia 1, 2,
- Junyu Chen 1, 2, *,
- Yixin Chen 1, 2, *
Author Information
Received: 09 Sep 2024 | Revised: 29 Sep 2024 | Accepted: 15 Oct 2024 | Published: 25 Oct 2024
Abstract
Graphical Abstract
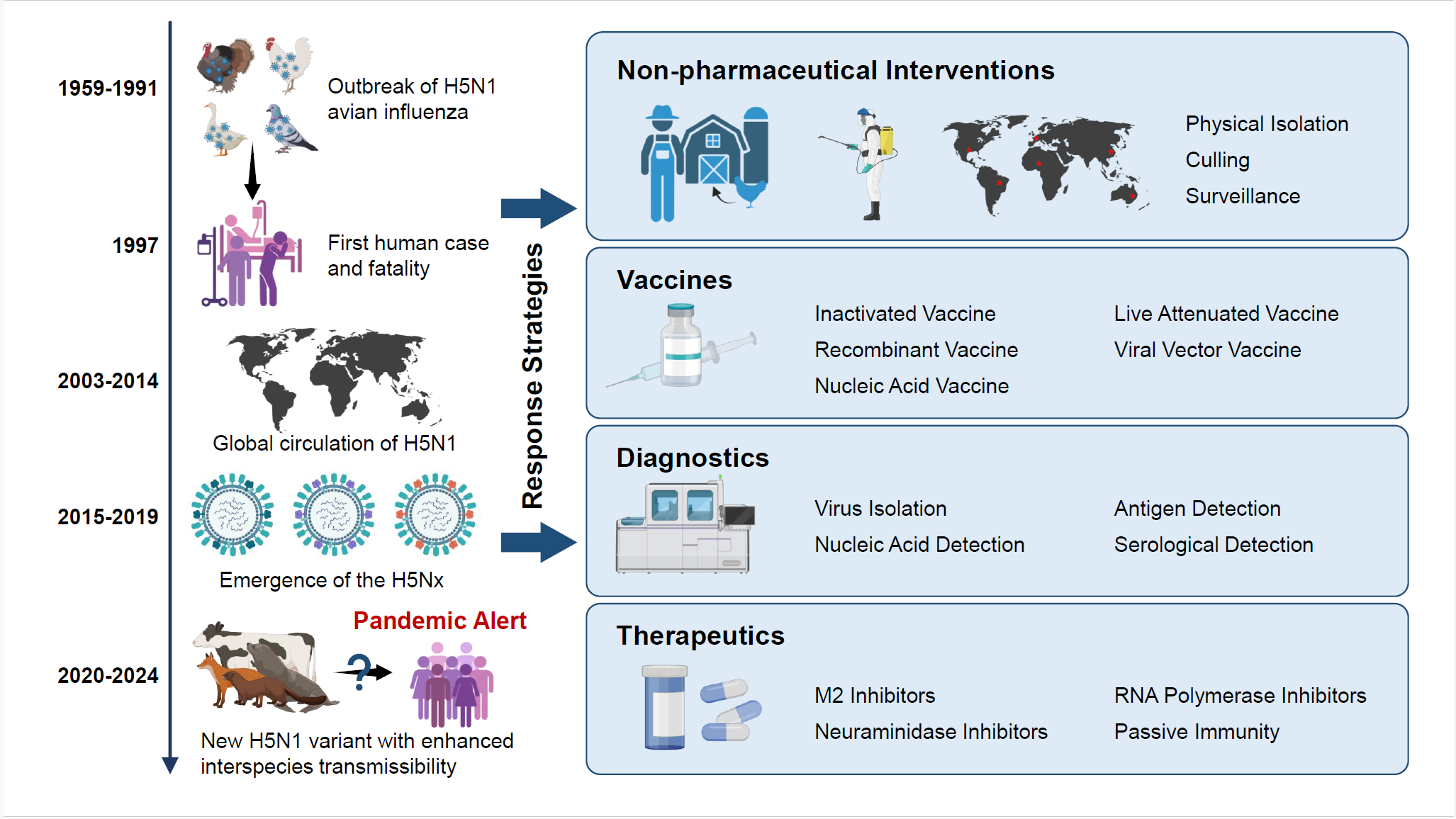
Keywords
H5N1 | circulation | vaccine development | diagnostics | treatment
References
- 1.Hu, X.; Saxena, A.; Magstadt, D.R.; Gauger, P.C.; Burrough, E.R.; Zhang, J.; Siepker, C.; Mainenti, M.; Gorden, P.J.; Plummer, P.J.; et al. Genomic characterization of highly pathogenic avian influenza A H5N1 virus newly emerged in dairy cattle. Emerg. Microbes Infect. 2024, 13, 2380421. https://doi.org/10.1080/22221751.2024.2380421.
- 2.H5 Bird Flu: Current Situation. Available online: https://www.cdc.gov/bird-flu/situation-summary/index.html (accessed on 4 October 2024).
- 3.Eisfeld, A.J.; Biswas, A.; Guan, L.; Gu, C.; Maemura, T.; Trifkovic, S.; Wang, T.; Babujee, L.; Dahn, R.; Halfmann, P.J.; et al. Pathogenicity and transmissibility of bovine H5N1 influenza virus. Nature 2024, 633, 426–432. https://doi.org/10.1038/s41586-024-07766-6.
- 4.Forni, D.; Cagliani, R.; Pozzoli, U.; Mozzi, A.; Arrigoni, F.; De Gioia, L.; Clerici, M.; Sironi, M. Dating the Emergence of Human Endemic Coronaviruses. Viruses 2022, 14, 11. https://doi.org/10.3390/v14051095.
- 5.Li, C.; Bu, Z.; Chen, H. Avian influenza vaccines against H5N1 'bird flu'. Trends Biotechnol. 2014, 32, 147–156. https://doi.org/10.1016/j.tibtech.2014.01.001.
- 6.Kim, J.H.; Cho, C.H.; Shin, J.H.; Yang, J.C.; Park, T.J.; Park, J.; Park, J.P. Highly sensitive and label-free detection of influenza H5N1 viral proteins using affinity peptide and porous BSA/MXene nanocomposite electrode. Anal. Chim. Acta 2023, 1251, 341018. https://doi.org/10.1016/j.aca.2023.341018.
- 7.Liu, J.; Xiao, H.; Lei, F.; Zhu, Q.; Qin, K.; Zhang, X.W.; Zhang, X.L.; Zhao, D.; Wang, G.; Feng, Y.; et al. Highly pathogenic H5N1 influenza virus infection in migratory birds. Science 2005, 309, 1206. https://doi.org/10.1126/science.1115273.
- 8.High Pathogenicity Avian Influenza (HPAI)—Situation Report 61. Available online: https://www.woah.org/en/document/high-pathogenicity-avian-influenza-hpai-situation-report-61/ (accessed on 28 August 2024).
- 9.2020–2024 Highlights in the History of Avian Influenza (Bird Flu) Timeline. Available online: https://www.cdc.gov/bird-flu/avian-timeline/2020s.html?CDC_AAref_Val=https://www.cdc.gov/flu/avianflu/timeline/avian-timeline-2020s.htm (accessed on 28 August 2024).
- 10.Avian Influenza A (H5N1)—Australia. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON519 (accessed on 28 August 2024).
- 11.Pereira, H.G.; Tůmová, B.; Law, V.G. Avian influenza A viruses. Bull. World Health Organ. 1965, 32, 855–860.
- 12.Alexander, D.J.; Lister, S.A.; Johnson, M.J.; Randall, C.J.; Thomas, P.J. An outbreak of highly pathogenic avian influenza in turkeys in Great Britain in 1991. Vet. Rec. 1993, 132, 535–536. https://doi.org/10.1136/vr.132.21.535.
- 13.Xu, X.; Subbarao; Cox, N.J.; Guo, Y. Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: Similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology 1999, 261, 15–19. https://doi.org/10.1006/viro.1999.9820.
- 14.Subbarao, K.; Klimov, A.; Katz, J.; Regnery, H.; Lim, W.; Hall, H.; Perdue, M.; Swayne, D.; Bender, C.; Huang, J.; et al. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science 1998, 279, 393–396. https://doi.org/10.1126/science.279.5349.393.
- 15.Guan, Y.; Peiris, J.S.; Lipatov, A.S.; Ellis, T.M.; Dyrting, K.C.; Krauss, S.; Zhang, L.J.; Webster, R.G.; Shortridge, K.F. Emergence of multiple genotypes of H5N1 avian influenza viruses in Hong Kong SAR. Proc. Natl. Acad. Sci. USA 2002, 99, 8950–8955. https://doi.org/10.1073/pnas.132268999.
- 16.Alexander, D.J. Report on avian influenza in the Eastern Hemisphere during 1997–2002. Avian Dis. 2003, 47, 792–797. https://doi.org/10.1637/0005-2086-47.s3.792.
- 17.Guan, Y.; Poon, L.L.; Cheung, C.Y.; Ellis, T.M.; Lim, W.; Lipatov, A.S.; Chan, K.H.; Sturm-Ramirez, K.M.; Cheung, C.L.; Leung, Y.H.; et al. H5N1 influenza: A protean pandemic threat. Proc. Natl. Acad. Sci. USA 2004, 101, 8156–8161. https://doi.org/10.1073/pnas.0402443101.
- 18.Zhu, Q.Y.; Qin, E.D.; Wang, W.; Yu, J.; Liu, B.H.; Hu, Y.; Hu, J.F.; Cao, W.C. Fatal infection with influenza A (H5N1) virus in China. N. Engl. J. Med. 2006, 354, 2731–2732. https://doi.org/10.1056/NEJMc066058.
- 19.Tran, T.H.; Nguyen, T.L.; Nguyen, T.D.; Luong, T.S.; Pham, P.M.; Nguyenv, V.; Pham, T.S.; Vo, C.D.; Le, T.Q.; Ngo, T.T.; et al. Avian influenza A (H5N1) in 10 patients in Vietnam. N. Engl. J. Med. 2004, 350, 1179–1188. https://doi.org/10.1056/NEJMoa040419.
- 20.Chotpitayasunondh, T.; Ungchusak, K.; Hanshaoworakul, W.; Chunsuthiwat, S.; Sawanpanyalert, P.; Kijphati, R.; Lochindarat, S.; Srisan, P.; Suwan, P.; Osotthanakorn, Y.; et al. Human disease from influenza A (H5N1), Thailand, 2004. Emerg. Infect. Dis. 2005, 11, 201–209. https://doi.org/10.3201/eid1102.041061.
- 21.Chen, H.; Li, Y.; Li, Z.; Shi, J.; Shinya, K.; Deng, G.; Qi, Q.; Tian, G.; Fan, S.; Zhao, H.; et al. Properties and dissemination of H5N1 viruses isolated during an influenza outbreak in migratory waterfowl in western China. J. Virol. 2006, 80, 5976–5983. https://doi.org/10.1128/jvi.00110-06.
- 22.Chen, H.; Smith, G.J.D.; Zhang, S.Y.; Qin, K.; Wang, J.; Li, K.S.; Webster, R.G.; Peiris, J.S.M.; Guan, Y. H5N1 virus outbreak in migratory waterfowl. Nature 2005, 436, 191–192. https://doi.org/10.1038/nature03974.
- 23.Global Statistics of Avian Influenza (As of 26 August 2024). Available online: https://www.chp.gov.hk/files/pdf/global_statistics_avian_influenza_e.pdf (accessed on 26 August 2024).
- 24.Naddaf, M. How the current bird flu strain evolved to be so deadly. Nature 2023, 622, 676–677. https://doi.org/10.1038/d41586-023-03256-3.
- 25.CDC. 2010–2019 Highlights in the History of Avian Influenza (Bird Flu) Timeline. Available online: https://www.cdc.gov/bird-flu/avian-timeline/2010-2019.html#cdcreference_1 (accessed on 30 August 2024).
- 26.Lewis, N.S.; Banyard, A.C.; Whittard, E.; Karibayev, T.; Al Kafagi, T.; Chvala, I.; Byrne, A.; Meruyert Akberovna, S.; King, J.; Harder, T.; et al. Emergence and spread of novel H5N8, H5N5 and H5N1 clade 2.3.4.4 highly pathogenic avian influenza in 2020. Emerg. Microbes Infect. 2021, 10, 148–151. https://doi.org/10.1080/22221751.2021.1872355.
- 27.Cui, P.; Shi, J.; Wang, C.; Zhang, Y.; Xing, X.; Kong, H.; Yan, C.; Zeng, X.; Liu, L.; Tian, G.; et al. Global dissemination of H5N1 influenza viruses bearing the clade 2.3.4.4b HA gene and biologic analysis of the ones detected in China. Emerg. Microbes Infect. 2022, 11, 1693–1704. https://doi.org/10.1080/22221751.2022.2088407.
- 28.Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Niqueux, É.; Staubach, C.; Terregino, C.; Aznar, I.; Muñoz Guajardo, I.; et al. Avian influenza overview December 2021–March 2022. Efsa j 2022, 20, e07289. https://doi.org/10.2903/j.efsa.2022.7289.
- 29.Bevins, S.N.; Shriner, S.A.; Cumbee, J.C., Jr.; Dilione, K.E.; Douglass, K.E.; Ellis, J.W.; Killian, M.L.; Torchetti, M.K.; Lenoch, J.B. Intercontinental Movement of Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4 Virus to the United States, 2021. Emerg. Microbes Infect. 2022, 28, 1006–1011. https://doi.org/10.3201/eid2805.220318.
- 30.Caserta, L.C.; Frye, E.A.; Butt, S.L.; Laverack, M.; Nooruzzaman, M.; Covaleda, L.M.; Thompson, A.C.; Koscielny, M.P.; Cronk, B.; Johnson, A.; et al. Spillover of highly pathogenic avian influenza H5N1 virus to dairy cattle. Nature 2024, 634, 669–676. https://doi.org/10.1038/s41586-024-07849-4.
- 31.Agüero, M.; Monne, I.; Sánchez, A.; Zecchin, B.; Fusaro, A.; Ruano, M.J.; Del Valle Arrojo, M.; Fernández-Antonio, R.; Souto, A.M.; Tordable, P.; et al. Highly pathogenic avian influenza A(H5N1) virus infection in farmed minks, Spain, October 2022. Eurosurveillance 2023, 28, 2300001. https://doi.org/10.2807/1560-7917.Es.2023.28.3.2300001.
- 32.Gamarra-Toledo, V.; Plaza, P.I.; Gutiérrez, R.; Inga-Diaz, G.; Saravia-Guevara, P.; Pereyra-Meza, O.; Coronado-Flores, E.; Calderón-Cerrón, A.; Quiroz-Jiménez, G.; Martinez, P.; et al. Mass Mortality of Sea Lions Caused by Highly Pathogenic Avian Influenza A(H5N1) Virus. Emerg. Infect. Dis. 2023, 29, 2553–2556. https://doi.org/10.3201/eid2912.230192.
- 33.Centers for Disease Control and Prevention, United States. A(H5N1) Bird Flu Response Update.July 19,2024. Available online: https://www.cdc.gov/bird-flu/spotlights/h5n1-response-07192024.html (accessed on 20 August 2024).
- 34.Sims, L.D.; Ellis, T.M.; Liu, K.K.; Dyrting, K.; Wong, H.; Peiris, M.; Guan, Y.; Shortridge, K.F. Avian influenza in Hong Kong 1997-2002. Avian Dis. 2003, 47, 832–838. https://doi.org/10.1637/0005-2086-47.s3.832.
- 35.World Health Organization (WHO); Food and Agriculture Organization (FAO). H5N1 highly pathogenic avian influenza: Timeline of major events. Available online: https://cdn.who.int/media/docs/default-source/influenza/avian-and-other-zoonotic-influenza/h5n1_avian_influenza_update20141204.pdf (accessed on 4 August 2024).
- 36.Brown, I.H. Summary of avian influenza activity in Europe, Asia, and Africa, 2006-2009. Avian Dis. 2010, 54, 187–193. https://doi.org/10.1637/8949-053109-Reg.1.
- 37.Manabe, T.; Yamaoka, K.; Tango, T.; Binh, N.G.; Co, D.X.; Tuan, N.D.; Izumi, S.; Takasaki, J.; Chau, N.Q.; Kudo, K. Chronological, geographical, and seasonal trends of human cases of avian influenza A (H5N1) in Vietnam, 2003-2014: A spatial analysis. BMC Infect. Dis. 2016, 16, 64. https://doi.org/10.1186/s12879-016-1391-8.
- 38.Charostad, J.; Rezaei Zadeh Rukerd, M.; Mahmoudvand, S.; Bashash, D.; Hashemi, S.M.A.; Nakhaie, M.; Zandi, K. A comprehensive review of highly pathogenic avian influenza (HPAI) H5N1: An imminent threat at doorstep. Travel. Med. Infect. Dis. 2023, 55, 102638. https://doi.org/10.1016/j.tmaid.2023.102638.
- 39.Park, Y.R.; Lee, Y.N.; Lee, D.H.; Baek, Y.G.; Si, Y.J.; Meeduangchanh, P.; Theppangna, W.; Douangngeun, B.; Kye, S.J.; Lee, M.H.; et al. Genetic and pathogenic characteristics of clade 2.3.2.1c H5N1 highly pathogenic avian influenza viruses isolated from poultry outbreaks in Laos during 2015–2018. Transbound Emerg. Dis. 2020, 67, 947–955. https://doi.org/10.1111/tbed.13430.
- 40.Arafa, A.S.; Naguib, M.M.; Luttermann, C.; Selim, A.A.; Kilany, W.H.; Hagag, N.; Samy, A.; Abdelhalim, A.; Hassan, M.K.; Abdelwhab, E.M.; et al. Emergence of a novel cluster of influenza A(H5N1) virus clade 2.2.1.2 with putative human health impact in Egypt, 2014/15. Eurosurveillance 2015, 20, 21805. https://doi.org/10.2807/1560-7917.es2015.20.13.21085.
- 41.Chen, H.; Smith, G.J.; Li, K.S.; Wang, J.; Fan, X.H.; Rayner, J.M.; Vijaykrishna, D.; Zhang, J.X.; Zhang, L.J.; Guo, C.T.; et al. Establishment of multiple sublineages of H5N1 influenza virus in Asia: Implications for pandemic control. Proc. Natl. Acad. Sci. USA 2006, 103, 2845–2850. https://doi.org/10.1073/pnas.0511120103.
- 42.World Health Organization (WHO); World Organisation for Animal Health (OIE); Food and Agriculture Organization (FAO). H5N1 Evolution Working Group. Revised and updated nomenclature for highly pathogenic avian influenza A (H5N1) viruses. Influenza Other Respir. Viruses 2014, 8, 384–388. https://doi.org/10.1111/irv.12230.
- 43.Toward a unified nomenclature system for highly pathogenic avian influenza virus (H5N1). Emerg. Infect. Dis. 2008, 14, e1. https://doi.org/10.3201/eid1407.071681.
- 44.Li, Y.; Li, M.; Li, Y.; Tian, J.; Bai, X.; Yang, C.; Shi, J.; Ai, R.; Chen, W.; Zhang, W.; et al. Outbreaks of Highly Pathogenic Avian Influenza (H5N6) Virus Subclade 2.3.4.4h in Swans, Xinjiang, Western China, 2020. Emerg. Infect. Dis. 2020, 26, 2956–2960. https://doi.org/10.3201/eid2612.201201.
- 45.Cui, Y.; Li, Y.; Li, M.; Zhao, L.; Wang, D.; Tian, J.; Bai, X.; Ci, Y.; Wu, S.; Wang, F.; et al. Evolution and extensive reassortment of H5 influenza viruses isolated from wild birds in China over the past decade. Emerg. Microbes Infect. 2020, 9, 1793–1803. https://doi.org/10.1080/22221751.2020.1797542.
- 46.Role for migratory wild birds in the global spread of avian influenza H5N8. Science 2016, 354, 213–217. https://doi.org/10.1126/science.aaf8852.
- 47.Artois, J.; Newman, S.H.; Dhingra, M.S.; Chaiban, C.; Linard, C.; Cattoli, G.; Monne, I.; Fusaro, A.; Xenarios, I.; Engler, R.; et al. Clade-level Spatial Modelling of HPAI H5N1 Dynamics in the Mekong Region Reveals New Patterns and Associations with Agro-Ecological Factors. Sci. Rep. 2016, 6, 30316. https://doi.org/10.1038/srep30316.
- 48.Wan, X.F.; Nguyen, T.; Davis, C.T.; Smith, C.B.; Zhao, Z.M.; Carrel, M.; Inui, K.; Do, H.T.; Mai, D.T.; Jadhao, S.; et al. Evolution of highly pathogenic H5N1 avian influenza viruses in Vietnam between 2001 and 2007. PLoS ONE 2008, 3, e3462. https://doi.org/10.1371/journal.pone.0003462.
- 49.Continuing progress towards a unified nomenclature for the highly pathogenic H5N1 avian influenza viruses: Divergence of clade 2.2 viruses. Influenza Other Respir. Viruses 2009, 3, 59–62. https://doi.org/10.1111/j.1750-2659.2009.00078.x.
- 50.Li, Y.; Liu, L.; Zhang, Y.; Duan, Z.; Tian, G.; Zeng, X.; Shi, J.; Zhang, L.; Chen, H. New avian influenza virus (H5N1) in wild birds, Qinghai, China. Emerg. Infect. Dis. 2011, 17, 265–267. https://doi.org/10.3201/eid1702.100732.
- 51.Zhou, J.; Sun, W.; Wang, J.; Guo, J.; Yin, W.; Wu, N.; Li, L.; Yan, Y.; Liao, M.; Huang, Y.; et al. Characterization of the H5N1 highly pathogenic avian influenza virus derived from wild pikas in China. J. Virol. 2009, 83, 8957–8964. https://doi.org/10.1128/jvi.00793-09.
- 52.Jiang, W.; Hou, G.; Li, J.; Peng, C.; Wang, S.; Chen, J. Novel variants of clade 2.3.2.1 H5N1 highly pathogenic avian influenza virus in migratory waterfowl of Hongze Lake. Vet. Microbiol. 2017, 198, 99–103. https://doi.org/10.1016/j.vetmic.2016.12.011.
- 53.Lee, D.H.; Bertran, K.; Kwon, J.H.; Swayne, D.E. Evolution, global spread, and pathogenicity of highly pathogenic avian influenza H5Nx clade 2.3.4.4. J. Vet. Sci. 2017, 18, 269–280. https://doi.org/10.4142/jvs.2017.18.S1.269.
- 54.Lee, D.H.; Torchetti, M.K.; Winker, K.; Ip, H.S.; Song, C.S.; Swayne, D.E. Intercontinental Spread of Asian-Origin H5N8 to North America through Beringia by Migratory Birds. J. Virol. 2015, 89, 6521–6524. https://doi.org/10.1128/jvi.00728-15.
- 55.Globig, A.; Starick, E.; Homeier, T.; Pohlmann, A.; Grund, C.; Wolf, P.; Zimmermann, A.; Wolf, C.; Heim, D.; Schlößer, H.; et al. Epidemiological and Molecular Analysis of an Outbreak of Highly Pathogenic Avian Influenza H5N8 clade 2.3.4.4 in a German Zoo: Effective Disease Control with Minimal Culling. Transbound. Emerg. Dis. 2017, 64, 1813–1824. https://doi.org/10.1111/tbed.12570.
- 56.Wille, M.; Barr, I.G. Resurgence of avian influenza virus. Science 2022, 376, 459–460. https://doi.org/10.1126/science.abo1232.
- 57.Alexakis, L.; Fusaro, A.; Kuiken, T.; Mirinavičiūtė, G.; Ståhl, K.; Staubach, C.; Svartström, O.; Terregino, C.; Willgert, K.; Delacourt, R.; et al. Avian influenza overview March-June 2024. EFSA j 2024, 22, e8930. https://doi.org/10.2903/j.efsa.2024.8930.
- 58.Sagong, M.; Lee, Y.N.; Song, S.; Cha, R.M.; Lee, E.K.; Kang, Y.M.; Cho, H.K.; Kang, H.M.; Lee, Y.J.; Lee, K.N. Emergence of clade 2.3.4.4b novel reassortant H5N1 high pathogenicity avian influenza virus in South Korea during late 2021. Transbound Emerg Dis 2022, 69, e3255–e3260. https://doi.org/10.1111/tbed.14551.
- 59.Kayano, T.; Kobayashi, T.; Fujiwara, S.; Okada, Y.; Nishiura, H. Survey of exposure to stranded dolphins in Japan to investigate an outbreak of suspected infection with highly pathogenic avian influenza (H5N1) clade 2.3.4.4(b) in humans. New Microbes New Infect. 2024, 56, 101214. https://doi.org/10.1016/j.nmni.2023.101214.
- 60.Abolnik, C.; Stutchbury, S.; Hartman, M.J. Experimental infection of racing pigeons (Columba livia domestica) with highly pathogenic Clade 2.3.4.4 sub-group B H5N8 avian influenza virus. Vet. Microbiol. 2018, 227, 127–132. https://doi.org/10.1016/j.vetmic.2018.10.028.
- 61.Joint FAO/WHO/WOAH preliminary assessment of recent influenza A(H5N1) viruses. Available online: https://www.who.int/publications/m/item/joint-fao-who-woah-preliminary-assessment-of-recent-influenza-a(h5n1)-viruses (accessed on 4 October 2024).
- 62.Lewis, N.; Beer, M. Stop H5N1 influenza in US cattle now. Science 2024, 385, 123. https://doi.org/10.1126/science.adr5866.
- 63.Xu, R.; Ekiert, D.C.; Krause, J.C.; Hai, R.; Crowe, J.E., Jr.; Wilson, I.A. Structural basis of preexisting immunity to the 2009 H1N1 pandemic influenza virus. Science 2010, 328, 357–360. https://doi.org/10.1126/science.1186430.
- 64.Scheibner, D.; Salaheldin, A.H.; Bagato, O.; Zaeck, L.M.; Mostafa, A.; Blohm, U.; Müller, C.; Eweas, A.F.; Franzke, K.; Karger, A.; et al. Phenotypic effects of mutations observed in the neuraminidase of human origin H5N1 influenza A viruses. PLoS Pathog. 2023, 19, e1011135. https://doi.org/10.1371/journal.ppat.1011135.
- 65.Krammer, F.; Schultz-Cherry, S. We need to keep an eye on avian influenza. Nat. Rev. Immunol. 2023, 23, 267–268. https://doi.org/10.1038/s41577-023-00868-8.
- 66.Swayne, D.E.; Spackman, E.; Pantin-Jackwood, M. Success factors for avian influenza vaccine use in poultry and potential impact at the wild bird-agricultural interface. Ecohealth 2014, 11, 94–108. https://doi.org/10.1007/s10393-013-0861-3.
- 67.Subbarao, K.; Chen, H.; Swayne, D.; Mingay, L.; Fodor, E.; Brownlee, G.; Xu, X.; Lu, X.; Katz, J.; Cox, N.; et al. Evaluation of a genetically modified reassortant H5N1 influenza A virus vaccine candidate generated by plasmid-based reverse genetics. Virology 2003, 305, 192–200. https://doi.org/10.1006/viro.2002.1742.
- 68.Zeng, X.-y.; He, X.-w.; Meng, F.; Ma, Q.; Wang, Y.; Bao, H.-m.; Liu, Y.-j.; Deng, G.-h.; Shi, J.-z.; Li, Y.-b.; et al. Protective efficacy of an H5/H7 trivalent inactivated vaccine (H5-Re13, H5-Re14, and H7-Re4 strains) in chickens, ducks, and geese against newly detected H5N1, H5N6, H5N8, and H7N9 viruses. J. Integr. Agric. 2022, 21, 2086–2094. https://doi.org/10.1016/S2095-3119(22)63904-2.
- 69.Zeng, X.-y.; Chen, X.-h.; Ma, S.-j.; Wu, J.-j.; Bao, H.-m.; Pan, S.-x.; Liu, Y.-j.; Deng, G.-h.; Shi, J.-z.; Chen, P.-c.; et al. Protective efficacy of an H5/H7 trivalent inactivated vaccine produced from Re-11, Re-12, and H7-Re2 strains against challenge with different H5 and H7 viruses in chickens. J. Integr. Agric. 2020, 19, 2294–2300. https://doi.org/10.1016/S2095-3119(20)63301-9.
- 70.Influenza Virus Vaccine, H5N1 (for National Stockpile). Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/influenza-virus-vaccine-h5n1-national-stockpile (accessed on 10 August 2024).
- 71.Carter, N.J.; Plosker, G.L. Prepandemic influenza vaccine H5N1 (split virion, inactivated, adjuvanted) [Prepandrix]: A review of its use as an active immunization against influenza A subtype H5N1 virus. BioDrugs 2008, 22, 279–292. https://doi.org/10.2165/00063030-200822050-00001.
- 72.Felberbaum, R.S. The baculovirus expression vector system: A commercial manufacturing platform for viral vaccines and gene therapy vectors. Biotechnol. J. 2015, 10, 702–714. https://doi.org/10.1002/biot.201400438.
- 73.Park, J.; Fong Legaspi, S.L.; Schwartzman, L.M.; Gygli, S.M.; Sheng, Z.M.; Freeman, A.D.; Matthews, L.M.; Xiao, Y.; Ramuta, M.D.; Batchenkova, N.A.; et al. An inactivated multivalent influenza A virus vaccine is broadly protective in mice and ferrets. Sci. Transl. Med. 2022, 14, eabo2167. https://doi.org/10.1126/scitranslmed.abo2167.
- 74.Baz, M.; Luke, C.J.; Cheng, X.; Jin, H.; Subbarao, K. H5N1 vaccines in humans. Virus Res. 2013, 178, 78–98. https://doi.org/10.1016/j.virusres.2013.05.006.
- 75.Zost, S.J.; Parkhouse, K.; Gumina, M.E.; Kim, K.; Diaz Perez, S.; Wilson, P.C.; Treanor, J.J.; Sant, A.J.; Cobey, S.; Hensley, S.E. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc. Natl. Acad. Sci. USA 2017, 114, 12578–12583. https://doi.org/10.1073/pnas.1712377114.
- 76.Treanor, J.J.; Campbell, J.D.; Zangwill, K.M.; Rowe, T.; Wolff, M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N. Engl. J. Med. 2006, 354, 1343–1351. https://doi.org/10.1056/NEJMoa055778.
- 77.Lamb, Y.N. Cell-Based Quadrivalent Inactivated Influenza Virus Vaccine (Flucelvax(®) Tetra/Flucelvax Quadrivalent(®)): A Review in the Prevention of Influenza. Drugs 2019, 79, 1337–1348. https://doi.org/10.1007/s40265-019-01176-z.
- 78.Plosker, G.L. A/H5N1 prepandemic influenza vaccine (whole virion, vero cell-derived, inactivated) [Vepacel®]. Drugs 2012, 72, 1543–1557. https://doi.org/10.2165/11209650-000000000-00000.
- 79.Li, S.; Liu, C.; Klimov, A.; Subbarao, K.; Perdue, M.L.; Mo, D.; Ji, Y.; Woods, L.; Hietala, S.; Bryant, M. Recombinant influenza A virus vaccines for the pathogenic human A/Hong Kong/97 (H5N1) viruses. J. Infect. Dis. 1999, 179, 1132–1138. https://doi.org/10.1086/314713.
- 80.Lee, Y.H.; Jang, Y.H.; Seong, B.L. Cell-cultured, live attenuated, X-31ca-based H5N1 pre-pandemic influenza vaccine. Virology 2017, 504, 73–78. https://doi.org/10.1016/j.virol.2017.01.021.
- 81.Nicolodi, C.; Groiss, F.; Kiselev, O.; Wolschek, M.; Seipelt, J.; Muster, T. Safety and immunogenicity of a replication-deficient H5N1 influenza virus vaccine lacking NS1. Vaccine 2019, 37, 3722–3729. https://doi.org/10.1016/j.vaccine.2019.05.013.
- 82.Ren, W.; Pei, S.; Jiang, W.; Zhao, M.; Jiang, L.; Liu, H.; Yi, Y.; Hui, M.; Li, J. A replication-deficient H9N2 influenza virus carrying H5 hemagglutinin conferred protection against H9N2 and H5N1 influenza viruses in mice. Front. Microbiol. 2022, 13, 1042916. https://doi.org/10.3389/fmicb.2022.1042916.
- 83.Treanor, J.J.; Wilkinson, B.E.; Masseoud, F.; Hu-Primmer, J.; Battaglia, R.; O'Brien, D.; Wolff, M.; Rabinovich, G.; Blackwelder, W.; Katz, J.M. Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in humans. Vaccine 2001, 19, 1732–1737. https://doi.org/10.1016/s0264-410x(00)00395-9.
- 84.Levine, M.Z.; Holiday, C.; Liu, F.; Jefferson, S.; Gillis, E.; Bellamy, A.R.; Tumpey, T.; Katz, J.M. Cross-Reactive Antibody Responses to Novel H5Nx Influenza Viruses Following Homologous and Heterologous Prime-Boost Vaccination with a Prepandemic Stockpiled A(H5N1) Vaccine in Humans. J. Infect. Dis. 2017, 216, S555–S559. https://doi.org/10.1093/infdis/jix001.
- 85.Hu, J.; Peng, P.; Li, J.; Zhang, Q.; Li, R.; Wang, X.; Gu, M.; Hu, Z.; Hu, S.; Liu, X.; et al. Single Dose of Bivalent H5 and H7 Influenza Virus-Like Particle Protects Chickens Against Highly Pathogenic H5N1 and H7N9 Avian Influenza Viruses. Front. Vet. Sci. 2021, 8, 774630. https://doi.org/10.3389/fvets.2021.774630.
- 86.Kong, D.; He, Y.; Wang, J.; Chi, L.; Ao, X.; Ye, H.; Qiu, W.; Zhu, X.; Liao, M.; Fan, H. A single immunization with H5N1 virus-like particle vaccine protects chickens against divergent H5N1 influenza viruses and vaccine efficacy is determined by adjuvant and dosage. Emerg. Microbes Infect. 2024, 13, 2287682. https://doi.org/10.1080/22221751.2023.2287682.
- 87.Ward, B.J.; Makarkov, A.; Séguin, A.; Pillet, S.; Trépanier, S.; Dhaliwall, J.; Libman, M.D.; Vesikari, T.; Landry, N. Efficacy, immunogenicity, and safety of a plant-derived, quadrivalent, virus-like particle influenza vaccine in adults (18-64 years) and older adults (≥65 years): Two multicentre, randomised phase 3 trials. Lancet 2020, 396, 1491–1503. https://doi.org/10.1016/s0140-6736(20)32014-6.
- 88.Ward, B.J.; Séguin, A.; Couillard, J.; Trépanier, S.; Landry, N. Phase III: Randomized observer-blind trial to evaluate lot-to-lot consistency of a new plant-derived quadrivalent virus like particle influenza vaccine in adults 18-49 years of age. Vaccine 2021, 39, 1528–1533. https://doi.org/10.1016/j.vaccine.2021.01.004.
- 89.Pushko, P.; Tretyakova, I.; Hidajat, R.; Sun, X.; Belser, J.A.; Tumpey, T.M. Multi-clade H5N1 virus-like particles: Immunogenicity and protection against H5N1 virus and effects of beta-propiolactone. Vaccine 2018, 36, 4346–4353. https://doi.org/10.1016/j.vaccine.2018.05.092.
- 90.Song, J.-M.; Hossain, J.; Yoo, D.-G.; Lipatov, A.S.; Davis, C.T.; Quan, F.-S.; Chen, L.-M.; Hogan, R.J.; Donis, R.O.; Compans, R.W.; et al. Protective immunity against H5N1 influenza virus by a single dose vaccination with virus-like particles. Virology 2010, 405, 165–175. https://doi.org/10.1016/j.virol.2010.05.034.
- 91.Kapczynski, D.R.; Tumpey, T.M.; Hidajat, R.; Zsak, A.; Chrzastek, K.; Tretyakova, I.; Pushko, P. Vaccination with virus-like particles containing H5 antigens from three H5N1 clades protects chickens from H5N1 and H5N8 influenza viruses. Vaccine 2016, 34, 1575–1581. https://doi.org/10.1016/j.vaccine.2016.02.011.
- 92.Abdel-Motal, U.M.; Guay, H.M.; Wigglesworth, K.; Welsh, R.M.; Galili, U. Immunogenicity of influenza virus vaccine is increased by anti-gal-mediated targeting to antigen-presenting cells. J. Virol. 2007, 81, 9131–9141. https://doi.org/10.1128/jvi.00647-07.
- 93.Ramamurthy, M.; Sankar, S.; Abraham, A.M.; Nandagopal, B.; Sridharan, G. B cell epitopes in the intrinsically disordered regions of neuraminidase and hemagglutinin proteins of H5N1 and H9N2 avian influenza viruses for peptide-based vaccine development. J. Cell Biochem. 2019, 120, 17534–17544. https://doi.org/10.1002/jcb.29017.
- 94.Oftung, F.; Næss, L.M.; Laake, I.; Stoloff, G.; Pleguezuelos, O. FLU-v, a Broad-Spectrum Influenza Vaccine, Induces Cross-Reactive Cellular Immune Responses in Humans Measured by Dual IFN-γ and Granzyme B ELISpot Assay. Vaccines 2022, 10, 1528. https://doi.org/10.3390/vaccines10091528.
- 95.Taylor, J.; Weinberg, R.; Kawaoka, Y.; Webster, R.G.; Paoletti, E. Protective immunity against avian influenza induced by a fowlpox virus recombinant. Vaccine 1988, 6, 504–508. https://doi.org/10.1016/0264-410X(88)90101-6.
- 96.Qiao, C.L.; Yu, K.Z.; Jiang, Y.P.; Jia, Y.Q.; Tian, G.B.; Liu, M.; Deng, G.H.; Wang, X.R.; Meng, Q.W.; Tang, X.Y. Protection of chickens against highly lethal H5N1 and H7N1 avian influenza viruses with a recombinant fowlpox virus co-expressing H5 haemagglutinin and N1 neuraminidase genes. Avian Pathol. 2003, 32, 25–31. https://doi.org/10.1080/0307945021000070688.
- 97.Chen, H. Avian influenza vaccination: The experience in China. OIE Rev. Sci. Tech. 2009, 28, 267–274. https://doi.org/10.20506/rst.28.1.1860.
- 98.Kim, S.H.; Samal, S.K. Innovation in Newcastle Disease Virus Vectored Avian Influenza Vaccines. Viruses 2019, 11, 300. https://doi.org/10.3390/v11030300.
- 99.Ge, J.; Deng, G.; Wen, Z.; Tian, G.; Wang, Y.; Shi, J.; Wang, X.; Li, Y.; Hu, S.; Jiang, Y.; et al. Newcastle disease virus-based live attenuated vaccine completely protects chickens and mice from lethal challenge of homologous and heterologous H5N1 avian influenza viruses. J. Virol. 2007, 81, 150–158. https://doi.org/10.1128/jvi.01514-06.
- 100.Hein, R.; Koopman, R.; García, M.; Armour, N.; Dunn, J.R.; Barbosa, T.; Martinez, A. Review of Poultry Recombinant Vector Vaccines. Avian Dis. 2021, 65, 438–452. https://doi.org/10.1637/0005-2086-65.3.438.
- 101.Liu, J.; Chen, P.; Jiang, Y.; Wu, L.; Zeng, X.; Tian, G.; Ge, J.; Kawaoka, Y.; Bu, Z.; Chen, H. A duck enteritis virus-vectored bivalent live vaccine provides fast and complete protection against H5N1 avian influenza virus infection in ducks. J. Virol. 2011, 85, 10989–10998. https://doi.org/10.1128/jvi.05420-11.
- 102.Zou, Z.; Huang, K.; Wei, Y.; Chen, H.; Liu, Z.; Jin, M. Construction of a highly efficient CRISPR/Cas9-mediated duck enteritis virus-based vaccine against H5N1 avian influenza virus and duck Tembusu virus infection. Sci. Rep. 2017, 7, 1478. https://doi.org/10.1038/s41598-017-01554-1.
- 103.Hoelscher, M.A.; Garg, S.; Bangari, D.S.; Belser, J.A.; Lu, X.; Stephenson, I.; Bright, R.A.; Katz, J.M.; Mittal, S.K.; Sambhara, S. Development of adenoviral-vector-based pandemic influenza vaccine against antigenically distinct human H5N1 strains in mice. Lancet 2006, 367, 475–481. https://doi.org/10.1016/s0140-6736(06)68076-8.
- 104.Hoelscher, M.A.; Singh, N.; Garg, S.; Jayashankar, L.; Veguilla, V.; Pandey, A.; Matsuoka, Y.; Katz, J.M.; Donis, R.; Mittal, S.K.; et al. A broadly protective vaccine against globally dispersed clade 1 and clade 2 H5N1 influenza viruses. J. Infect. Dis. 2008, 197, 1185–1188. https://doi.org/10.1086/529522.
- 105.Holman, D.H.; Wang, D.; Raja, N.U.; Luo, M.; Moore, K.M.; Woraratanadharm, J.; Mytle, N.; Dong, J.Y. Multi-antigen vaccines based on complex adenovirus vectors induce protective immune responses against H5N1 avian influenza viruses. Vaccine 2008, 26, 2627–2639. https://doi.org/10.1016/j.vaccine.2008.02.053.
- 106.Tompkins, S.M.; Zhao, Z.S.; Lo, C.Y.; Misplon, J.A.; Liu, T.; Ye, Z.; Hogan, R.J.; Wu, Z.; Benton, K.A.; Tumpey, T.M.; et al. Matrix protein 2 vaccination and protection against influenza viruses, including subtype H5N1. Emerg. Infect. Dis. 2007, 13, 426–435. https://doi.org/10.3201/eid1303.061125.
- 107.Leung, H.C.; Chan, C.C.; Poon, V.K.; Zhao, H.J.; Cheung, C.Y.; Ng, F.; Huang, J.D.; Zheng, B.J. An H5N1-based matrix protein 2 ectodomain tetrameric peptide vaccine provides cross-protection against lethal infection with H7N9 influenza virus. Emerg. Microbes Infect. 2015, 4, e22. https://doi.org/10.1038/emi.2015.22.
- 108.Sayedahmed, E.E.; Hassan, A.O.; Kumari, R.; Cao, W.; Gangappa, S.; York, I.; Sambhara, S.; Mittal, S.K. A Bovine Adenoviral Vector-Based H5N1 Influenza -Vaccine Provides Enhanced Immunogenicity and Protection at a Significantly Low Dose. Mol. Ther. Methods Clin. Dev. 2018, 10, 210–222. https://doi.org/10.1016/j.omtm.2018.07.007.
- 109.Rao, S.S.; Kong, W.P.; Wei, C.J.; Van Hoeven, N.; Gorres, J.P.; Nason, M.; Andersen, H.; Tumpey, T.M.; Nabel, G.J. Comparative efficacy of hemagglutinin, nucleoprotein, and matrix 2 protein gene-based vaccination against H5N1 influenza in mouse and ferret. PLoS ONE 2010, 5, e9812. https://doi.org/10.1371/journal.pone.0009812.
- 110.A Phase I, Dose-Ranging Safety and Immunogenicity Study of an Adenovirus-vectored Intranasal, Pandemic (Hemagglutinin H5) Influenza Vaccine, ADhVN1203/04.H5, in Healthy Adults. Available online: https://clinicaltrials.gov/study/NCT00755703 (accessed on 4 October 2024).
- 111.Khurana, S.; Coyle, E.M.; Manischewitz, J.; King, L.R.; Ishioka, G.; Alexander, J.; Smith, J.; Gurwith, M.; Golding, H. Oral priming with replicating adenovirus serotype 4 followed by subunit H5N1 vaccine boost promotes antibody affinity maturation and expands H5N1 cross-clade neutralization. PLoS ONE 2015, 10, e0115476. https://doi.org/10.1371/journal.pone.0115476.
- 112.Li, Z.; Gabbard, J.D.; Mooney, A.; Gao, X.; Chen, Z.; Place, R.J.; Tompkins, S.M.; He, B. Single-Dose Vaccination of a Recombinant Parainfluenza Virus 5 Expressing NP from H5N1 Virus Provides Broad Immunity against Influenza A Viruses. J. Virol. 2013, 87, 5985–5993. https://doi.org/10.1128/jvi.00120-13.
- 113.Chen, J.; Wang, P.; Yuan, L.; Zhang, L.; Zhang, L.; Zhao, H.; Chen, C.; Wang, X.; Han, J.; Chen, Y.; et al. A live attenuated virus-based intranasal COVID-19 vaccine provides rapid, prolonged, and broad protection against SARS-CoV-2. Sci. Bull. 2022, 67, 1372–1387. https://doi.org/10.1016/j.scib.2022.05.018.
- 114.Chen, J.; Chen, C.; Yuan, L.; Chen, Y.; Wang, X.; Tang, N.; Wei, D.; Ye, X.; Xia, N.; Chen, Y. Intranasal influenza-vectored COVID-19 vaccines confer broad protection against SARS-CoV-2 XBB variants in hamsters. PNAS Nexus 2024, 3, pgae183. https://doi.org/10.1093/pnasnexus/pgae183.
- 115.Pardi, N.; Parkhouse, K.; Kirkpatrick, E.; McMahon, M.; Zost, S.J.; Mui, B.L.; Tam, Y.K.; Karikó, K.; Barbosa, C.J.; Madden, T.D.; et al. Nucleoside-modified mRNA immunization elicits influenza virus hemagglutinin stalk-specific antibodies. Nat. Commun. 2018, 9, 3361. https://doi.org/10.1038/s41467-018-05482-0.
- 116.Furey, C.; Scher, G.; Ye, N.; Kercher, L.; DeBeauchamp, J.; Crumpton, J.C.; Jeevan, T.; Patton, C.; Franks, J.; Rubrum, A.; et al. Development of a nucleoside-modified mRNA vaccine against clade 2.3.4.4b H5 highly pathogenic avian influenza virus. Nat. Commun. 2024, 15, 4350. https://doi.org/10.1038/s41467-024-48555-z.
- 117.Kodihalli, S.; Goto, H.; Kobasa, D.L.; Krauss, S.; Kawaoka, Y.; Webster, R.G. DNA Vaccine Encoding Hemagglutinin Provides Protective Immunity against H5N1 Influenza Virus Infection in Mice. J. Virol. 1999, 73, 2094–2098, doi:doi:10.1128/jvi.73.3.2094-2098.1999.
- 118.Jiang, Y.; Yu, K.; Zhang, H.; Zhang, P.; Li, C.; Tian, G.; Li, Y.; Wang, X.; Ge, J.; Bu, Z.; et al. Enhanced protective efficacy of H5 subtype avian influenza DNA vaccine with codon optimized HA gene in a pCAGGS plasmid vector. Antivir. Res. 2007, 75, 234–241. https://doi.org/10.1016/j.antiviral.2007.03.009.
- 119.Ma, Q.; Mu, Y.; Gong, L.; Zhu, C.; Di, S.; Cheng, M.; Gao, J.; Shi, J.; Zhang, L. Manganese-based nanoadjuvants for enhancement of immune effect of DNA vaccines. Front. Bioeng. Biotechnol. 2022, 10, 1053872. https://doi.org/10.3389/fbioe.2022.1053872.
- 120.Swayne, D.E. Avian influenza vaccines and therapies for poultry. Comp. Immunol. Microbiol. Infect. Dis. 2009, 32, 351–363. https://doi.org/10.1016/j.cimid.2008.01.006.
- 121.Tian, G.; Zhang, S.; Li, Y.; Bu, Z.; Liu, P.; Zhou, J.; Li, C.; Shi, J.; Yu, K.; Chen, H. Protective efficacy in chickens, geese and ducks of an H5N1-inactivated vaccine developed by reverse genetics. Virology 2005, 341, 153–162. https://doi.org/10.1016/j.virol.2005.07.011.
- 122.Li, N.; Du, S.; Wang, Y.; Zhu, X.; Shu, S.; Men, Y.; He, M.; Fang, F.; Wang, Y.; Gong, Y.; et al. Randomized, double-blinded, placebo-controlled phase I study of the pharmacokinetics, pharmacodynamics, and safety of KL130008, a novel oral JAK inhibitor, in healthy subjects. Eur. J. Pharm. Sci. 2022, 176, 106257. https://doi.org/10.1016/j.ejps.2022.106257.
- 123.Duong, T.N.; Thiem, V.D.; Anh, D.D.; Cuong, N.P.; Thang, T.C.; Huong, V.M.; Chien, V.C.; Phuong, N.T.L.; Montomoli, E.; Holt, R.; et al. A Phase 2/3 double blinded, randomized, placebo-controlled study in healthy adult participants in Vietnam to examine the safety and immunogenicity of an inactivated whole virion, alum adjuvanted, A(H5N1) influenza vaccine (IVACFLU-A/H5N1). Vaccine 2020, 38, 1541–1550. https://doi.org/10.1016/j.vaccine.2019.11.059.
- 124.Lazarus, R.; Kelly, S.; Snape, M.D.; Vandermeulen, C.; Voysey, M.; Hoppenbrouwers, K.; Hens, A.; Van Damme, P.; Pepin, S.; Leroux-Roels, I.; et al. Antibody Persistence and Booster Responses to Split-Virion H5N1 Avian Influenza Vaccine in Young and Elderly Adults. PLoS ONE 2016, 11, e0165384. https://doi.org/10.1371/journal.pone.0165384.
- 125.Cargnin Faccin, F.; Perez, D.R. Pandemic preparedness through vaccine development for avian influenza viruses. Hum. Vaccin. Immunother. 2024, 20, 2347019. https://doi.org/10.1080/21645515.2024.2347019.
- 126.Izurieta, P.; Kim, W.J.; Wie, S.H.; Lee, J.; Lee, J.S.; Dramé, M.; Vaughn, D.W.; Schuind, A. Immunogenicity and safety of an AS03-adjuvanted H5N1 pandemic influenza vaccine in Korean adults: A phase IV, randomized, open-label, controlled study. Vaccine 2015, 33, 2800–2807. https://doi.org/10.1016/j.vaccine.2015.04.027.
- 127.Frey, S.S.; Versage, E.; Van Twuijver, E.; Hohenboken, M. Antibody responses against heterologous H5N1 strains for an MF59-adjuvanted cell culture-derived H5N1 (aH5n1c) influenza vaccine in adults and older adults. Hum. Vaccin. Immunother. 2023, 19, 2193119. https://doi.org/10.1080/21645515.2023.2193119.
- 128.Hill-Batorski, L.; Hatta, Y.; Moser, M.J.; Sarawar, S.; Neumann, G.; Kawaoka, Y.; Bilsel, P. Quadrivalent Formulation of Intranasal Influenza Vaccine M2SR (M2-Deficient Single Replication) Protects against Drifted Influenza A and B Virus Challenge. Vaccines 2023, 11, 798. https://doi.org/10.3390/vaccines11040798.
- 129.Rudenko, L.; Desheva, J.; Korovkin, S.; Mironov, A.; Rekstin, A.; Grigorieva, E.; Donina, S.; Gambaryan, A.; Katlinsky, A. Safety and immunogenicity of live attenuated influenza reassortant H5 vaccine (phase I-II clinical trials). Influenza Other Respir. Viruses 2008, 2, 203–209. https://doi.org/10.1111/j.1750-2659.2008.00064.x.
- 130.Atsmon, J.; Kate-Ilovitz, E.; Shaikevich, D.; Singer, Y.; Volokhov, I.; Haim, K.Y.; Ben-Yedidia, T. Safety and Immunogenicity of Multimeric-001—A Novel Universal Influenza Vaccine. J. Clin. Immunol. 2012, 32, 595–603. https://doi.org/10.1007/s10875-011-9632-5.
- 131.Treanor, J.J.; Essink, B.; Hull, S.; Reed, S.; Izikson, R.; Patriarca, P.; Goldenthal, K.L.; Kohberger, R.; Dunkle, L.M. Evaluation of safety and immunogenicity of recombinant influenza hemagglutinin (H5/Indonesia/05/2005) formulated with and without a stable oil-in-water emulsion containing glucopyranosyl-lipid A (SE+GLA) adjuvant. Vaccine 2013, 31, 5760–5765. https://doi.org/10.1016/j.vaccine.2013.08.064.
- 132.Ge, P.; Ross, T.M. COBRA HA and NA vaccination elicits long-live protective immune responses against pre-pandemic H2, H5, and H7 influenza virus subtypes. Virology 2024, 597, 110119. https://doi.org/10.1016/j.virol.2024.110119.
- 133.Pillet, S.; Aubin, É.; Trépanier, S.; Poulin, J.F.; Yassine-Diab, B.; Ter Meulen, J.; Ward, B.J.; Landry, N. Humoral and cell-mediated immune responses to H5N1 plant-made virus-like particle vaccine are differentially impacted by alum and GLA-SE adjuvants in a Phase 2 clinical trial. NPJ Vaccines 2018, 3, 3. https://doi.org/10.1038/s41541-017-0043-3.
- 134.Landry, N.; Ward, B.J.; Trépanier, S.; Montomoli, E.; Dargis, M.; Lapini, G.; Vézina, L.P. Preclinical and clinical development of plant-made virus-like particle vaccine against avian H5N1 influenza. PLoS ONE 2010, 5, e15559. https://doi.org/10.1371/journal.pone.0015559.
- 135.Pavlova, S.P.; Veits, J.; Keil, G.M.; Mettenleiter, T.C.; Fuchs, W. Protection of chickens against H5N1 highly pathogenic avian influenza virus infection by live vaccination with infectious laryngotracheitis virus recombinants expressing H5 hemagglutinin and N1 neuraminidase. Vaccine 2009, 27, 773–785. https://doi.org/10.1016/j.vaccine.2008.11.033.
- 136.Mooney Alaina, J.; Gabbard Jon, D.; Li, Z.; Dlugolenski Daniel, A.; Johnson Scott, K.; Tripp Ralph, A.; He, B.; Tompkins, S.M. Vaccination with Recombinant Parainfluenza Virus 5 Expressing Neuraminidase Protects against Homologous and Heterologous Influenza Virus Challenge. J. Virol. 2017, 91, 10.1128/jvi.01579-01517. https://doi.org/10.1128/jvi.01579-17.
- 137.Bull, M.B.; Ma, F.N.; Perera, L.P.; Poon, L.L.; Valkenburg, S.A. Early vaccine-mediated strain-specific cytokine imbalance induces mild immunopathology during influenza infection. Immunol. Cell Biol. 2023, 101, 514–524. https://doi.org/10.1111/imcb.12608.
- 138.Scallan, C.D.; Lindbloom, J.D.; Tucker, S.N. Oral Modeling of an Adenovirus-Based Quadrivalent Influenza Vaccine in Ferrets and Mice. Infect. Dis. Ther. 2016, 5, 165–183. https://doi.org/10.1007/s40121-016-0108-z.
- 139.Xu, Z.; Wise, M.C.; Chokkalingam, N.; Walker, S.; Tello-Ruiz, E.; Elliott, S.T.C.; Perales-Puchalt, A.; Xiao, P.; Zhu, X.; Pumroy, R.A.; et al. In Vivo Assembly of Nanoparticles Achieved through Synergy of Structure-Based Protein Engineering and Synthetic DNA Generates Enhanced Adaptive Immunity. Adv Sci (Weinh) 2020, 7, 1902802. https://doi.org/10.1002/advs.201902802.
- 140.WHO. Causality Assessment of an Adverse Event Following Immunization (AEFI) User Manual for the Revised WHO Classification. 2019. Available online: https://iris.who.int/bitstream/handle/10665/340802/9789241516990-eng.pdf?sequence=1 (accessed on 10 October 2024).
- 141.Hong, S.C.; Murale, D.P.; Jang, S.Y.; Haque, M.M.; Seo, M.; Lee, S.; Woo, D.H.; Kwon, J.; Song, C.S.; Kim, Y.K.; et al. Discrimination of Avian Influenza Virus Subtypes using Host-Cell Infection Fingerprinting by a Sulfinate-based Fluorescence Superoxide Probe. Angew. Chem. Int. Ed. Engl. 2018, 57, 9716–9721. https://doi.org/10.1002/anie.201804412.
- 142.Sakurai, A.; Shibasaki, F. Updated values for molecular diagnosis for highly pathogenic avian influenza virus. Viruses 2012, 4, 1235–1257. https://doi.org/10.3390/v4081235.
- 143.Avelin, V.; Sissonen, S.; Julkunen, I.; Österlund, P. Inactivation efficacy of H5N1 avian influenza virus by commonly used sample preparation reagents for safe laboratory practices. J. Virol. Methods 2022, 304, 114527. https://doi.org/10.1016/j.jviromet.2022.114527.
- 144.Steininger, C.; Kundi, M.; Aberle, S.W.; Aberle, J.H.; Popow-Kraupp, T. Effectiveness of reverse transcription-PCR, virus isolation, and enzyme-linked immunosorbent assay for diagnosis of influenza A virus infection in different age groups. J. Clin. Microbiol. 2002, 40, 2051–2056. https://doi.org/10.1128/jcm.40.6.2051-2056.2002.
- 145.Wei, H.L.; Bai, G.R.; Mweene, A.S.; Zhou, Y.C.; Cong, Y.L.; Pu, J.; Wang, S.; Kida, H.; Liu, J.H. Rapid detection of avian influenza virus a and subtype H5N1 by single step multiplex reverse transcription-polymerase chain reaction. Virus Genes. 2006, 32, 261–267. https://doi.org/10.1007/s11262-005-6910-4.
- 146.Payungporn, S.; Phakdeewirot, P.; Chutinimitkul, S.; Theamboonlers, A.; Keawcharoen, J.; Oraveerakul, K.; Amonsin, A.; Poovorawan, Y. Single-step multiplex reverse transcription-polymerase chain reaction (RT-PCR) for influenza A virus subtype H5N1 detection. Viral Immunol. 2004, 17, 588–593. https://doi.org/10.1089/vim.2004.17.588.
- 147.Bustin, S.A.; Benes, V.; Nolan, T.; Pfaffl, M.W. Quantitative real-time RT-PCR--a perspective. J. Mol. Endocrinol. 2005, 34, 597–601. https://doi.org/10.1677/jme.1.01755.
- 148.Chen, W.; He, B.; Li, C.; Zhang, X.; Wu, W.; Yin, X.; Fan, B.; Fan, X.; Wang, J. Real-time RT-PCR for H5N1 avian influenza A virus detection. J. Med. Microbiol. 2007, 56, 603–607. https://doi.org/10.1099/jmm.0.47014-0.
- 149.Payungporn, S.; Chutinimitkul, S.; Chaisingh, A.; Damrongwantanapokin, S.; Buranathai, C.; Amonsin, A.; Theamboonlers, A.; Poovorawan, Y. Single step multiplex real-time RT-PCR for H5N1 influenza A virus detection. J. Virol. Methods 2006, 131, 143–147. https://doi.org/10.1016/j.jviromet.2005.08.004.
- 150.Moore, C.; Telles, J.N.; Corden, S.; Gao, R.B.; Vernet, G.; Van Aarle, P.; Shu, Y.L. Development and validation of a commercial real-time NASBA assay for the rapid confirmation of influenza A H5N1 virus in clinical samples. J. Virol. Methods 2010, 170, 173–176. https://doi.org/10.1016/j.jviromet.2010.09.014.
- 151.McMullen, A.R.; Anderson, N.W.; Burnham, C.A. Pathology Consultation on Influenza Diagnostics. Am. J. Clin. Pathol. 2016, 145, 440–448. https://doi.org/10.1093/ajcp/aqw039.
- 152.Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. https://doi.org/10.1093/nar/28.12.e63.
- 153.Imai, M.; Ninomiya, A.; Minekawa, H.; Notomi, T.; Ishizaki, T.; Van Tu, P.; Tien, N.T.; Tashiro, M.; Odagiri, T. Rapid diagnosis of H5N1 avian influenza virus infection by newly developed influenza H5 hemagglutinin gene-specific loop-mediated isothermal amplification method. J. Virol. Methods 2007, 141, 173–180. https://doi.org/10.1016/j.jviromet.2006.12.004.
- 154.Imai, M.; Ninomiya, A.; Minekawa, H.; Notomi, T.; Ishizaki, T.; Tashiro, M.; Odagiri, T. Development of H5-RT-LAMP (loop-mediated isothermal amplification) system for rapid diagnosis of H5 avian influenza virus infection. Vaccine 2006, 24, 6679–6682. https://doi.org/10.1016/j.vaccine.2006.05.046.
- 155.Jayawardena, S.; Cheung, C.Y.; Barr, I.; Chan, K.H.; Chen, H.; Guan, Y.; Peiris, J.S.; Poon, L.L. Loop-mediated isothermal amplification for influenza A (H5N1) virus. Emerg. Infect. Dis. 2007, 13, 899–901. https://doi.org/10.3201/eid1306.061572.
- 156.Piepenburg, O.; Williams, C.H.; Stemple, D.L.; Armes, N.A. DNA detection using recombination proteins. PLoS Biol. 2006, 4, e204. https://doi.org/10.1371/journal.pbio.0040204.
- 157.Yehia, N.; Arafa, A.S.; Abd El Wahed, A.; El-Sanousi, A.A.; Weidmann, M.; Shalaby, M.A. Development of reverse transcription recombinase polymerase amplification assay for avian influenza H5N1 HA gene detection. J. Virol. Methods 2015, 223, 45–49. https://doi.org/10.1016/j.jviromet.2015.07.011.
- 158.Sangsiriwut, K.; Uiprasertkul, M.; Payungporn, S.; Auewarakul, P.; Ungchusak, K.; Chantratita, W.; Puthavathana, P. Complete Genomic Sequences of Highly Pathogenic H5N1 Avian Influenza Viruses Obtained Directly from Human Autopsy Specimens. Microbiol. Resour. Announc. 2018, 7. https://doi.org/10.1128/mra.01498-18.
- 159.Domańska-Blicharz, K.; Świętoń, E.; Świątalska, A.; Monne, I.; Fusaro, A.; Tarasiuk, K.; Wyrostek, K.; Styś-Fijoł, N.; Giza, A.; Pietruk, M.; et al. Outbreak of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus in cats, Poland, June to July 2023. Eurosurveillance 2023, 28, 2300366. https://doi.org/10.2807/1560-7917.Es.2023.28.31.2300366.
- 160.Hou, S.Y.; Chen, H.K.; Cheng, H.C.; Huang, C.Y. Development of zeptomole and attomolar detection sensitivity of biotin-peptide using a dot-blot gold nanoparticle immunoassay. Anal. Chem. 2007, 79, 980–985. https://doi.org/10.1021/ac061507g.
- 161.Peng, F.; Wang, Z.; Zhang, S.; Wu, R.; Hu, S.; Li, Z.; Wang, X.; Bi, D. Development of an immunochromatographic strip for rapid detection of H9 subtype avian influenza viruses. Clin. Vaccine Immunol. 2008, 15, 569–574. https://doi.org/10.1128/cvi.00273-07.
- 162.Preechakasedkit, P.; Pinwattana, K.; Dungchai, W.; Siangproh, W.; Chaicumpa, W.; Tongtawe, P.; Chailapakul, O. Development of a one-step immunochromatographic strip test using gold nanoparticles for the rapid detection of Salmonella typhi in human serum. Biosens. Bioelectron. 2012, 31, 562–566. https://doi.org/10.1016/j.bios.2011.10.031.
- 163.Taban, F.; Rapiti, E.; Fioretta, G.; Wespi, Y.; Weintraub, D.; Hugli, A.; Schubert, H.; Vlastos, G.; Castiglione, M.; Bouchardy, C. Breast cancer management and outcome according to surgeon's affiliation: A population-based comparison adjusted for patient's selection bias. Ann. Oncol. 2013, 24, 116–125. https://doi.org/10.1093/annonc/mds285.
- 164.Cui, S.; Tong, G. A chromatographic strip test for rapid detection of one lineage of the H5 subtype of highly pathogenic avian influenza. J. Vet. Diagn. Invest. 2008, 20, 567–571. https://doi.org/10.1177/104063870802000505.
- 165.Li, R.; Li, P.; Guo, X.; Jin, M.; Zhang, W.; Zhang, Q. A Chromatographic Strip for Rapid Semi-quantitative Detection of H5 Subtype Avian Influenza Viruses in Poultry. Food Anal. Methods 2013, 6, 1712–1717. https://doi.org/10.1007/s12161-013-9606-8.
- 166.Tsuda, Y.; Sakoda, Y.; Sakabe, S.; Mochizuki, T.; Namba, Y.; Kida, H. Development of an immunochromatographic kit for rapid diagnosis of H5 avian influenza virus infection. Microbiol. Immunol. 2007, 51, 903–907. https://doi.org/10.1111/j.1348-0421.2007.tb03973.x.
- 167.Sakurai, A.; Takayama, K.; Nomura, N.; Munakata, T.; Yamamoto, N.; Tamura, T.; Yamada, J.; Hashimoto, M.; Kuwahara, K.; Sakoda, Y.; et al. Broad-spectrum detection of H5 subtype influenza A viruses with a new fluorescent immunochromatography system. PLoS ONE 2013, 8, e76753. https://doi.org/10.1371/journal.pone.0076753.
- 168.Wada, A.; Sakoda, Y.; Oyamada, T.; Kida, H. Development of a highly sensitive immunochromatographic detection kit for H5 influenza virus hemagglutinin using silver amplification. J. Virol. Methods 2011, 178, 82–86. https://doi.org/10.1016/j.jviromet.2011.08.017.
- 169.Chen, Y.; Xu, F.; Fan, X.; Luo, H.; Ge, S.; Zheng, Q.; Xia, N.; Chen, H.; Guan, Y.; Zhang, J. Evaluation of a rapid test for detection of H5N1 avian influenza virus. J. Virol. Methods 2008, 154, 213–215. https://doi.org/10.1016/j.jviromet.2008.08.013.
- 170.Chen, Y.; Xu, F.; Gui, X.; Yang, K.; Wu, X.; Zheng, Q.; Ge, S.; Yuan, Q.; Yeo, A.E.; Zhang, J.; et al. A rapid test for the detection of influenza A virus including pandemic influenza A/H1N1 2009. J. Virol. Methods 2010, 167, 100–102. https://doi.org/10.1016/j.jviromet.2010.02.001.
- 171.Yin, J.; Liu, S.; Zhu, Y. An overview of the highly pathogenic H5N1 influenza virus. Virol. Sin. 2013, 28, 3–15. https://doi.org/10.1007/s12250-013-3294-9.
- 172.Pawar, S.D.; Parkhi, S.S.; Koratkar, S.S.; Mishra, A.C. Receptor specificity and erythrocyte binding preferences of avian influenza viruses isolated from India. Virol. J. 2012, 9, 251. https://doi.org/10.1186/1743-422x-9-251.
- 173.Bishai, F.R.; Galli, R. Enzyme-linked immunosorbent assay for detection of antibodies to influenza A and B and parainfluenza type 1 in sera of patients. J. Clin. Microbiol. 1978, 8, 648–656. https://doi.org/10.1128/jcm.8.6.648-656.1978.
- 174.Zhou, E.M.; Chan, M.; Heckert, R.A.; Riva, J.; Cantin, M.F. Evaluation of a competitive ELISA for detection of antibodies against avian influenza virus nucleoprotein. Avian Dis. 1998, 42, 517–522.
- 175.Fu, X.; Wang, Q.; Ma, B.; Zhang, B.; Sun, K.; Yu, X.; Ye, Z.; Zhang, M. Advances in Detection Techniques for the H5N1 Avian Influenza Virus. Int. J. Mol. Sci. 2023, 24, 17157. https://doi.org/10.3390/ijms242417157.
- 176.Wu, R.; Hu, S.; Xiao, Y.; Li, Z.; Shi, D.; Bi, D. Development of indirect enzyme-linked immunosorbent assay with nucleoprotein as antigen for detection and quantification of antibodies against avian influenza virus. Vet. Res. Commun. 2007, 31, 631–641. https://doi.org/10.1007/s11259-007-3510-x.
- 177.Stelzer-Braid, S.; Wong, B.; Robertson, P.; Lynch, G.W.; Laurie, K.; Shaw, R.; Barr, I.; Selleck, P.W.; Baleriola, C.; Escott, R.; et al. A commercial ELISA detects high levels of human H5 antibody but cross-reacts with influenza A antibodies. J. Clin. Virol. 2008, 43, 241–243. https://doi.org/10.1016/j.jcv.2008.06.012.
- 178.Sączyńska, V.; Florys-Jankowska, K.; Porębska, A.; Cecuda-Adamczewska, V. A novel epitope-blocking ELISA for specific and sensitive detection of antibodies against H5-subtype influenza virus hemagglutinin. Virol. J. 2021, 18, 91. https://doi.org/10.1186/s12985-021-01564-6.
- 179.Velumani, S.; Ho, H.T.; He, F.; Musthaq, S.; Prabakaran, M.; Kwang, J. A novel peptide ELISA for universal detection of antibodies to human H5N1 influenza viruses. PLoS ONE 2011, 6, e20737. https://doi.org/10.1371/journal.pone.0020737.
- 180.Crossley, B.M.; Rejmanek, D.; Baroch, J.; Stanton, J.B.; Young, K.T.; Killian, M.L.; Torchetti, M.K.; Hietala, S.K. Nanopore sequencing as a rapid tool for identification and pathotyping of avian influenza A viruses. J. Vet. Diagn. Invest. 2021, 33, 253–260. https://doi.org/10.1177/1040638720984114.
- 181.Pellegrini, F.; Buonavoglia, A.; Omar, A.H.; Diakoudi, G.; Lucente, M.S.; Odigie, A.E.; Sposato, A.; Augelli, R.; Camero, M.; Decaro, N.; et al. A Cold Case of Equine Influenza Disentangled with Nanopore Sequencing. Animals 2023, 13, 1153. https://doi.org/10.3390/ani13071153.
- 182.Abudayyeh, O.O.; Gootenberg, J.S.; Konermann, S.; Joung, J.; Slaymaker, I.M.; Cox, D.B.; Shmakov, S.; Makarova, K.S.; Semenova, E.; Minakhin, L.; et al. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science 2016, 353, aaf5573. https://doi.org/10.1126/science.aaf5573.
- 183.Zhou, X.; Wang, S.; Ma, Y.; Jiang, Y.; Li, Y.; Shi, J.; Deng, G.; Tian, G.; Kong, H.; Wang, X. On-Site and Visual Detection of the H5 Subtype Avian Influenza Virus Based on RT-RPA and CRISPR/Cas12a. Viruses 2024, 16, 753. https://doi.org/10.3390/v16050753.
- 184.Park, B.J.; Park, M.S.; Lee, J.M.; Song, Y.J. Specific Detection of Influenza A and B Viruses by CRISPR-Cas12a-Based Assay. Biosensors 2021, 11, 88. https://doi.org/10.3390/bios11030088.
- 185.Bai, H.; Wang, R.; Hargis, B.; Lu, H.; Li, Y. A SPR aptasensor for detection of avian influenza virus H5N1. Sensors 2012, 12, 12506–12518. https://doi.org/10.3390/s120912506.
- 186.Gao, A.; Lu, N.; Dai, P.; Li, T.; Pei, H.; Gao, X.; Gong, Y.; Wang, Y.; Fan, C. Silicon-nanowire-based CMOS-compatible field-effect transistor nanosensors for ultrasensitive electrical detection of nucleic acids. Nano Lett. 2011, 11, 3974–3978. https://doi.org/10.1021/nl202303y.
- 187.Kukol, A.; Li, P.; Estrela, P.; Ko-Ferrigno, P.; Migliorato, P. Label-free electrical detection of DNA hybridization for the example of influenza virus gene sequences. Anal. Biochem. 2008, 374, 143–153. https://doi.org/10.1016/j.ab.2007.10.035.
- 188.Wen, L.Y.; Xu, H.; Lan, Y.; Zhao, X.; Zhang, X.G.; Wang, D.Y.; Yao, L.H.; Dong, J.; Zhang, J.H.; Guo, Y.J.; et al. Development of methods for detection of H5N1 from human clinical specimens. Zhonghua Shi Yan He Lin. Chuang Bing. Du. Xue Za Zhi 2006, 20, 24–26.
- 189.Pasick, J. Advances in the molecular based techniques for the diagnosis and characterization of avian influenza virus infections. Transbound. Emerg. Dis. 2008, 55, 329–338. https://doi.org/10.1111/j.1865-1682.2008.01047.x.
- 190.Yehia, N.; Eldemery, F.; Arafa, A.S.; Abd El Wahed, A.; El Sanousi, A.; Weidmann, M.; Shalaby, M. Reverse Transcription Recombinase Polymerase Amplification Assay for Rapid Detection of Avian Influenza Virus H9N2 HA Gene. Vet. Sci. 2021, 8, 134. https://doi.org/10.3390/vetsci8070134.
- 191.Kessler, N.; Ferraris, O.; Palmer, K.; Marsh, W.; Steel, A. Use of the DNA flow-thru chip, a three-dimensional biochip, for typing and subtyping of influenza viruses. J. Clin. Microbiol. 2004, 42, 2173–2185. https://doi.org/10.1128/jcm.42.5.2173-2185.2004.
- 192.Briand, F.X.; Schmitz, A.; Ogor, K.; Le Prioux, A.; Guillou-Cloarec, C.; Guillemoto, C.; Allée, C.; Le Bras, M.O.; Hirchaud, E.; Quenault, H.; et al. Emerging highly pathogenic H5 avian influenza viruses in France during winter 2015/16: Phylogenetic analyses and markers for zoonotic potential. Eurosurveillance 2017, 22, 30473. https://doi.org/10.2807/1560-7917.Es.2017.2.9.30473.
- 193.Keitel, K.; Wagner, N.; Lacroix, L.; Manzano, S.; Gervaix, A. Performance characteristics of a rapid immunochromatographic assay for detection of pandemic influenza A (H1N1) virus in children. Eur. J. Pediatr. 2011, 170, 511–517. https://doi.org/10.1007/s00431-010-1326-0.
- 194.Zhao, W.; Zhang, W.P.; Zhang, Z.L.; He, R.L.; Lin, Y.; Xie, M.; Wang, H.Z.; Pang, D.W. Robust and highly sensitive fluorescence approach for point-of-care virus detection based on immunomagnetic separation. Anal. Chem. 2012, 84, 2358–2365. https://doi.org/10.1021/ac203102u.
- 195.Batool, S.; Chokkakula, S.; Song, M.S. Influenza Treatment: Limitations of Antiviral Therapy and Advantages of Drug Combination Therapy. Microorganisms 2023, 11, 183. https://doi.org/10.3390/microorganisms11010183.
- 196.Chow, E.J.; Doyle, J.D.; Uyeki, T.M. Influenza virus-related critical illness: Prevention, diagnosis, treatment. Crit. Care 2019, 23, 214. https://doi.org/10.1186/s13054-019-2491-9.
- 197.Hayden, F.G.; Sugaya, N.; Hirotsu, N.; Lee, N.; De Jong, M.D.; Hurt, A.C.; Ishida, T.; Sekino, H.; Yamada, K.; Portsmouth, S.; et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N. Engl. J. Med. 2018, 379, 913–923. https://doi.org/10.1056/NEJMoa1716197.
- 198.Taniguchi, K.; Ando, Y.; Kobayashi, M.; Toba, S.; Nobori, H.; Sanaki, T.; Noshi, T.; Kawai, M.; Yoshida, R.; Sato, A.; et al. Characterization of the In Vitro and In Vivo Efficacy of Baloxavir Marboxil against H5 Highly Pathogenic Avian Influenza Virus Infection. Viruses 2022, 14, 111. https://doi.org/10.3390/v14010111.
- 199.Eriksson, M.; Nylén, S.; Grönvik, K.O. Passive immunization of mice with IgY anti-H5N1 protects against experimental influenza virus infection and allows development of protective immunity. Vaccine 2024, 42, 126133. https://doi.org/10.1016/j.vaccine.2024.07.034.
- 200.Kong, L.K.; Zhou, B.P. Successful treatment of avian influenza with convalescent plasma. Hong Kong Med. J. 2006, 12, 489.
- 201.Zhou, B.; Zhong, N.; Guan, Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N. Engl. J. Med. 2007, 357, 1450–1451. https://doi.org/10.1056/NEJMc070359.
- 202.Rockman, S.; Lowther, S.; Camuglia, S.; Vandenberg, K.; Taylor, S.; Fabri, L.; Miescher, S.; Pearse, M.; Middleton, D.; Kent, S.J.; et al. Intravenous Immunoglobulin Protects Against Severe Pandemic Influenza Infection. EBioMedicine 2017, 19, 119–127. https://doi.org/10.1016/j.ebiom.2017.04.010.
- 203.Luke, T.C.; Casadevall, A.; Watowich, S.J.; Hoffman, S.L.; Beigel, J.H.; Burgess, T.H. Hark back: Passive immunotherapy for influenza and other serious infections. Crit. Care Med. 2010, 38, e66-73. https://doi.org/10.1097/CCM.0b013e3181d44c1e.
- 204.Tan, Y.; Ng, Q.; Jia, Q.; Kwang, J.; He, F. A novel humanized antibody neutralizes H5N1 influenza virus via two different mechanisms. J. Virol. 2015, 89, 3712–3722. https://doi.org/10.1128/JVI.03014-14.
- 205.Han, J.; Schmitz, A.J.; Richey, S.T.; Dai, Y.N.; Turner, H.L.; Mohammed, B.M.; Fremont, D.H.; Ellebedy, A.H.; Ward, A.B. Polyclonal epitope mapping reveals temporal dynamics and diversity of human antibody responses to H5N1 vaccination. Cell Rep. 2021, 34, 108682. https://doi.org/10.1016/j.celrep.2020.108682.
- 206.Chen, Y.; Qin, K.; Wu, W.L.; Li, G.; Zhang, J.; Du, H.; Ng, M.H.; Shih, J.W.; Peiris, J.S.; Guan, Y.; et al. Broad cross-protection against H5N1 avian influenza virus infection by means of monoclonal antibodies that map to conserved viral epitopes. J. Infect. Dis. 2009, 199, 49–58. https://doi.org/10.1086/594374.
- 207.Lin, Q.; Li, T.; Chen, Y.; Lau, S.Y.; Wei, M.; Zhang, Y.; Zhang, Z.; Yao, Q.; Li, J.; Li, Z.; et al. Structural Basis for the Broad, Antibody-Mediated Neutralization of H5N1 Influenza Virus. J. Virol. 2018, 92. https://doi.org/10.1128/jvi.00547-18.
- 208.Yang, Z.; Li, Z.; Zhan, Y.; Lin, Z.; Fang, Z.; Xu, X.; Lin, L.; Li, H.; Lin, Z.; Kang, C.; et al. Safety and efficacy of onradivir in adults with acute uncomplicated influenza A infection: A multicentre, double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Infect. Dis. 2024, 24, 535–545. https://doi.org/10.1016/s1473-3099(23)00743-0.
- 209.Li, Y.Y.; Liang, G.D.; Chen, Z.X.; Zhang, K.; Liang, J.L.; Jiang, L.R.; Yang, S.Z.; Jiang, F.; Liu, S.W.; Yang, J. A small molecule compound targeting hemagglutinin inhibits influenza A virus and exhibits broad-spectrum antiviral activity. Acta Pharmacol. Sin. 2024, 45, 2380–2393. https://doi.org/10.1038/s41401-024-01331-7.
- 210.Huang, L.; Wang, J.; Ma, X.; Sun, L.; Hao, C.; Wang, W. Inhibition of influenza a virus infection by natural stilbene piceatannol targeting virus hemagglutinin. Phytomedicine 2023, 120, 155058. https://doi.org/10.1016/j.phymed.2023.155058.
- 211.Lv, C.; Li, Y.; Wang, T.; Zhang, Q.; Qi, J.; Sima, M.; Li, E.; Qin, T.; Shi, Z.; Li, F.; et al. Taurolidine improved protection against highly pathogenetic avian influenza H5N1 virus lethal-infection in mouse model by regulating the NF-κB signaling pathway. Virol. Sin. 2023, 38, 119–127. https://doi.org/10.1016/j.virs.2022.11.010.
- 212.Schnell, J.R.; Chou, J.J. Structure and mechanism of the M2 proton channel of influenza A virus. Nature 2008, 451, 591–595. https://doi.org/10.1038/nature06531.
- 213.Stouffer, A.L.; Acharya, R.; Salom, D.; Levine, A.S.; Di Costanzo, L.; Soto, C.S.; Tereshko, V.; Nanda, V.; Stayrook, S.; DeGrado, W.F. Structural basis for the function and inhibition of an influenza virus proton channel. Nature 2008, 451, 596–599. https://doi.org/10.1038/nature06528.
- 214.Jefferson, T.; Demicheli, V.; Di Pietrantonj, C.; Rivetti, D. Amantadine and rimantadine for influenza A in adults. Cochrane Database Syst. Rev. 2006, 2006, Cd001169. https://doi.org/10.1002/14651858.CD001169.pub3.
- 215.Montalto, N.J.; Gum, K.D.; Ashley, J.V. Updated treatment for influenza A and B. Am. Fam. Physician 2000, 62, 2467–2476.
- 216.Aoki, F.Y.; Boivin, G.; Roberts, N. Influenza virus susceptibility and resistance to oseltamivir. Antivir. Ther. 2007, 12, 603–616.
- 217.Gao, Y.; Guyatt, G.; Uyeki, T.M.; Liu, M.; Chen, Y.; Zhao, Y.; Shen, Y.; Xu, J.; Zheng, Q.; Li, Z.; et al. Antivirals for treatment of severe influenza: A systematic review and network meta-analysis of randomised controlled trials. Lancet 2024, 404, 753–763. https://doi.org/10.1016/s0140-6736(24)01307-2.
- 218.Stiver, G. The treatment of influenza with antiviral drugs. Cmaj 2003, 168, 49–56.
- 219.Lee, N.; Hurt, A.C. Neuraminidase inhibitor resistance in influenza: A clinical perspective. Curr. Opin. Infect. Dis. 2018, 31, 520–526. https://doi.org/10.1097/qco.0000000000000498.
- 220.Scott, L.J. Peramivir: A Review in Uncomplicated Influenza. Drugs 2018, 78, 1363–1370. https://doi.org/10.1007/s40265-018-0981-8.
- 221.Ison, M.G. Antivirals and resistance: Influenza virus. Curr. Opin. Virol. 2011, 1, 563–573. https://doi.org/10.1016/j.coviro.2011.09.002.
- 222.Hayden, F.G.; Shindo, N. Influenza virus polymerase inhibitors in clinical development. Curr. Opin. Infect. Dis. 2019, 32, 176–186. https://doi.org/10.1097/qco.0000000000000532.
- 223.Hickerson, B.T.; Petrovskaya, S.N.; Dickensheets, H.; Donnelly, R.P.; Ince, W.L.; Ilyushina, N.A. Impact of Baloxavir Resistance-Associated Substitutions on Influenza Virus Growth and Drug Susceptibility. J. Virol. 2023, 97, e0015423. https://doi.org/10.1128/jvi.00154-23.
- 224.Krammer, F. The human antibody response to influenza A virus infection and vaccination. Nat. Rev. Immunol. 2019, 19, 383–397. https://doi.org/10.1038/s41577-019-0143-6.
- 225.Simmons, C.P.; Bernasconi, N.L.; Suguitan, A.L.; Mills, K.; Ward, J.M.; Chau, N.V.; Hien, T.T.; Sallusto, F.; Do Quang, H.; Farrar, J.; et al. Prophylactic and therapeutic efficacy of human monoclonal antibodies against H5N1 influenza. PLoS Med. 2007, 4, e178. https://doi.org/10.1371/journal.pmed.0040178.
- 226.Hanson, B.J.; Boon, A.C.; Lim, A.P.; Webb, A.; Ooi, E.E.; Webby, R.J. Passive immunoprophylaxis and therapy with humanized monoclonal antibody specific for influenza A H5 hemagglutinin in mice. Respir. Res. 2006, 7, 126. https://doi.org/10.1186/1465-9921-7-126.
- 227.Prabhu, N.; Prabakaran, M.; Hongliang, Q.; He, F.; Ho, H.T.; Qiang, J.; Goutama, M.; Lim, A.P.; Hanson, B.J.; Kwang, J. Prophylactic and therapeutic efficacy of a chimeric monoclonal antibody specific for H5 haemagglutinin against lethal H5N1 influenza. Antivir. Ther. 2009, 14, 911–921. https://doi.org/10.3851/imp1413.
- 228.Shen, C.; Chen, J.; Li, R.; Zhang, M.; Wang, G.; Stegalkina, S.; Zhang, L.; Chen, J.; Cao, J.; Bi, X.; et al. A multimechanistic antibody targeting the receptor binding site potently cross-protects against influenza B viruses. Sci. Transl. Med. 2017, 9, eaam5752. https://doi.org/10.1126/scitranslmed.aam5752.
- 229.Li, T.; Chen, J.; Zheng, Q.; Xue, W.; Zhang, L.; Rong, R.; Zhang, S.; Wang, Q.; Hong, M.; Zhang, Y.; et al. Identification of a cross-neutralizing antibody that targets the receptor binding site of H1N1 and H5N1 influenza viruses. Nat. Commun. 2022, 13, 5182. https://doi.org/10.1038/s41467-022-32926-5.
- 230.Luke, T.C.; Kilbane, E.M.; Jackson, J.L.; Hoffman, S.L. Meta-analysis: Convalescent blood products for Spanish influenza pneumonia: A future H5N1 treatment? Ann. Intern. Med. 2006, 145, 599–609. https://doi.org/10.7326/0003-4819-145-8-200610170-00139.
How to Cite
Wei, D.; Wu, X.; Chen, H.; Chen, K.; Xia, N.; Chen, J.; Chen, Y. H5N1 Avian Influenza: Global Circulation and Response Strategies. Health and Metabolism 2024, 1 (1), 2. https://doi.org/10.53941/hm.2024.100002.
RIS
BibTex
Copyright & License

Copyright (c) 2024 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


