Sjogren’s syndrome (SS) is one of the most common chronic autoimmune diseases primarily affecting the salivary and lacrimal glands, leading to dry mouth and dry eyes, with systemic involvement in severe cases. This article provides an overview of the pathogenic mechanisms underlying SS, including genetic predisposition, immune dysregulation, cytokine imbalances, autoantibody production, and metabolic alterations. Additionally, advancements in treatment strategies are discussed, ranging from symptomatic relief to targeted biological therapies, such as B-cell depletion and cytokine modulation. While significant progress has been made in understanding the pathological mechanisms of SS, challenges persist in disease classification, biomarker identification, and therapeutic development. Future research is needed to focus on refining diagnostic criteria and exploring novel therapeutic interventions to improve disease management and patient outcomes.
- Open Access
- Review
Pathogenic Mechanisms and Treatment Advancements of Sjogren’s Syndrome
- Derica C. Tang,
- Shen Hu *
Author Information
Received: 06 Apr 2025 | Revised: 25 Apr 2025 | Accepted: 21 May 2025 | Published: 27 May 2025
Abstract
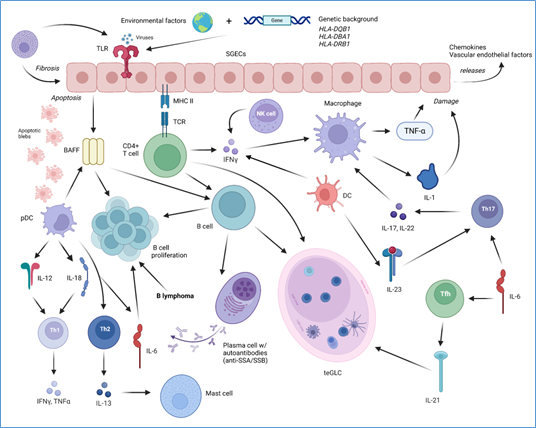
Graphical Abstract
Keywords
Sjogren’s syndrome | biomarkers | immunopathogenesis | B-cell activation | cytokine imbalance, autoantibodies | DNA methylation | immunotherapy
References
- 1.Negrini,; Emmi, G.; Greco, M.; Borro, M.; Sardanelli, F.; Murdaca, G.; Indiveri, F.; Puppo, F. Sjögren’s syndrome: A systemic autoimmune disease. Clin. Exp. Med. 2022, 22, 9–25. https://doi.org/10.1007/s10238-021-00728-6.
- 2.Sebastian,; Szachowicz, A.; Wiland, P. Classification criteria for secondary Sjögren’s syndrome. Current state of knowledge. Reumatologia 2019, 57, 277–280. https://doi.org/10.5114/reum.2019.89520.
- 3.Tarn,R.; Howard-Tripp, N.; Lendrem, D.W.; Mariette, X.; Saraux, A.; Devauchelle-Pensec, V.; Seror, R.; Skelton, A.J.; James, K.; McMeekin, P.; et al. Symptom-based stratification of patients with primary Sjögren’s syndrome: Multi-dimensional characterisation of international observational cohorts and reanalyses of randomised clinical trials. Lancet Rheumatol. 2019, 1, e85–e94. https://doi.org/10.1016/s2665-9913(19)30042-6.
- 4.Brito-Zerón,; Baldini, C.; Bootsma, H.; Bowman, S.J.; Jonsson, R.; Mariette, X.; Sivils, K.; Theander, E.; Tzioufas, A.; Ramos-Casals, M. Sjögren syndrome. Nat. Rev. Dis. Primers 2016, 2, 16047. https://doi.org/10.1038/nrdp.2016.47.
- 5.Taylor,E.; Wong, Q.; Levine, D.M.; McHugh, C.; Laurie, C.; Doheny, K.; Lam, M.Y.; Baer, A.N.; Challacombe, S.; Lanfranchi, H.; et al. Genome-Wide Association Analysis Reveals Genetic Heterogeneity of Sjogren’s Syndrome According to Ancestry. Arthritis Rheumatol. 2017, 69, 1294–1305. https://doi.org/10.1002/art.40040.
- 6.Ramos-Casals,; Tzioufas, A.G.; Stone, J.H.; Sisó, A.; Bosch, X. Treatment of primary Sjögren syndrome: A systematic review. JAMA 2010, 304, 452–460. https://doi.org/10.1001/jama.2010.1014.
- 7.Vitali,; Minniti, A.; Pignataro, F.; Maglione, W.; Del Papa, N. Management of Sjögren’s Syndrome: Present Issues and Future Perspectives. Front. Med. 2021, 8, 676885. https://doi.org/10.3389/fmed.2021.676885.
- 8.Qi,; Tian, J.; Wang, G.; Yan, Y.; Wang, T.; Wei, Y.; Wang, Z.; Zhang, G.; Zhang, Y.; Wang, J. Advances in cellular and molecular pathways of salivary gland damage in Sjogren’s syndrome. Front. Immunol. 2024, 15, 1405126. https://doi.org/10.3389/fimmu.2024.1405126.
- 9.Rizzo,; Grasso, G.; Destro Castaniti, G.M.; Ciccia, F.; Guggino, G. Primary Sjogren Syndrome: Focus on Innate Immune Cells and Inflammation. Vaccines 2020, 8, 272. https://doi.org/10.3390/vaccines8020272.
- 10.Carapito,; Gottenberg, J.-E.; Kotova, I.; Untrau, M.; Michel, S.; Naegely, L.; Aouadi, I.; Kwemou, M.; Paul, N.; Pichot, A.; et al. A new MHC-linked susceptibility locus for primary Sjögren’s syndrome: MICA. Hum. Mol. Genet. 2017, 26, 2565–2576. https://doi.org/10.1093/hmg/ddx135.
- 11.Nocturne,; Mariette, X. B cells in the pathogenesis of primary Sjögren syndrome. Nat. Rev. Rheumatol. 2018, 14, 133–145. https://doi.org/10.1038/nrrheum.2018.1.
- 12.Altan-Bonnet,; Mukherjee, R. Cytokine-mediated communication: A quantitative appraisal of immune complexity. Nat. Rev. Immunol. 2019, 19, 205–217. https://doi.org/10.1038/s41577-019-0131-x.
- 13.Patel,H.; Leone, R.D.; Horton, M.R.; Powell, J.D. Targeting metabolism to regulate immune responses in autoimmunity and cancer. Nat. Rev. Drug Discov. 2019, 18, 669–688. https://doi.org/10.1038/s41573-019-0032-5.
- 14.Pringle,; Wang, X.; Verstappen, G.; Terpstra, J.H.; Zhang, C.K.; He, A.; Patel, V.; Jones, R.E.; Baird, D.M.; Spijkervet, F.K.L.; et al. Salivary Gland Stem Cells Age Prematurely in Primary Sjögren’s Syndrome. Arthritis Rheumatol. 2019, 71, 133–142. https://doi.org/10.1002/art.40659.
- 15.Dalskov,; Gad, H.H.; Hartmann, R. Viral recognition and the antiviral interferon response. EMBO J. 2023, 42, e112907. https://doi.org/10.15252/embj.2022112907.
- 16.Soret,; Le Dantec, C.; Desvaux, E.; Foulquier, N.; Chassagnol, B.; Hubert, S.; Jamin, C.; Barturen, G.; Desachy, G.; Devauchelle-Pensec, V.; et al. A new molecular classification to drive precision treatment strategies in primary Sjögren’s syndrome. Nat. Commun. 2021, 12, 3523. https://doi.org/10.1038/s41467-021-23472-7.
- 17.Liu,; Yang, Y.; Zeng, L.; Wang, L.; He, C.; Chen, Z.; Sun, J.; Lyu, T.; Wang, M.; Chen, H.; et al. TOX promotes follicular helper T cell differentiation in patients with primary Sjögren’s syndrome. Rheumatology 2023, 62, 946–957. https://doi.org/10.1093/rheumatology/keac304.
- 18.Chao,C.; Lin, C.H.; Liao, T.L.; Chen, Y.M.; Chen, D.Y.; Chen, H.H. Association between a history of mycobacterial infection and the risk of newly diagnosed Sjögren’s syndrome: A nationwide, population-based case-control study. PLoS ONE 2017, 12, e0176549. https://doi.org/10.1371/journal.pone.0176549.
- 19.Silva,M.; Alves, C.E.C.; Pontes, G.S. Epstein-Barr virus: The mastermind of immune chaos. Front. Immunol. 2024, 15, 1297994. https://doi.org/10.3389/fimmu.2024.1297994.
- 20.Kwok,K.; Lee, J.; Yu, D.; Kang, K.Y.; Cho, M.L.; Kim, H.R.; Ju, J.H.; Lee, S.H.; Park, S.H.; Kim, H.Y. A pathogenetic role for IL-21 in primary Sjögren syndrome. Nat. Rev. Rheumatol. 2015, 11, 368–374. https://doi.org/10.1038/nrrheum.2014.225.
- 21.Nocturne,; Mariette, X. Advances in understanding the pathogenesis of primary Sjögren’s syndrome. Nat. Rev. Rheumatol. 2013, 9, 544–556. https://doi.org/10.1038/nrrheum.2013.110.
- 22.Zhan,; Zhang, J.; Lin, Y.; Chen, W.; Fan, X.; Zhang, D. Pathogenesis and treatment of Sjogren’s syndrome: Review and update. Front. Immunol. 2023, 14, 1127417. https://doi.org/10.3389/fimmu.2023.1127417.
- 23.Thorlacius,E.; Björk, A.; Wahren-Herlenius, M. Genetics and epigenetics of primary Sjögren syndrome: Implications for future therapies. Nat. Rev. Rheumatol. 2023, 19, 288–306. https://doi.org/10.1038/s41584-023-00932-6.
- 24.Zhao,; Jin, S.; Wang, S.; Zhang, Z.; Wang, X.; Chen, Z.; Wang, X.; Huang, S.; Zhang, D.; Wu, H. Tertiary lymphoid structures in diseases: Immune mechanisms and therapeutic advances. Signal Transduct. Target. Ther. 2024, 9, 225. https://doi.org/10.1038/s41392-024-01947-5.
- 25.Dong,; Wang, T.; Wu, H. Tertiary lymphoid structures in autoimmune diseases. Front. Immunol. 2023, 14, 1322035. https://doi.org/10.3389/fimmu.2023.1322035.
- 26.Nayar,; Campos, J.; Smith, C.G.; Iannizzotto, V.; Gardner, D.H.; Mourcin, F.; Roulois, D.; Turner, J.; Sylvestre, M.; Asam, S.; et al. Immunofibroblasts are pivotal drivers of tertiary lymphoid structure formation and local pathology. Proc. Natl. Acad. Sci. USA 2019, 116, 13490–13497. https://doi.org/10.1073/pnas.1905301116.
- 27.Elkon,; Casali, P. Nature and functions of autoantibodies. Nat. Clin. Pract. Rheumatol. 2008, 4, 491–498. https://doi.org/10.1038/ncprheum0895.
- 28.Du,; Han, M.; Zhu, X.; Xiao, F.; Huang, E.; Che, N.; Tang, X.; Zou, H.; Jiang, Q.; Lu, L. The Multiple Roles of B Cells in the Pathogenesis of Sjögren’s Syndrome. Front. Immunol. 2021, 12, 684999. https://doi.org/10.3389/fimmu.2021.684999.
- 29.Verstappen,M.; Pringle, S.; Bootsma, H.; Kroese, F.G.M. Epithelial-immune cell interplay in primary Sjögren syndrome salivary gland pathogenesis. Nat. Rev. Rheumatol. 2021, 17, 333–348. https://doi.org/10.1038/s41584-021-00605-2.
- 30.Birnbaum,; Hoke, A.; Lalji, A.; Calabresi, P.; Bhargava, P.; Casciola-Rosen, L. Brief Report: Anti-Calponin 3 Autoantibodies: A Newly Identified Specificity in Patients With Sjögren’s Syndrome. Arthritis Rheumatol. 2018, 70, 1610–1616. https://doi.org/10.1002/art.40550.
- 31.Soto-Heredero,; Gómez de Las Heras, M.M.; Gabandé-Rodríguez, E.; Oller, J.; Mittelbrunn, M. Glycolysis—A key player in the inflammatory response. FEBS J. 2020, 287, 3350–3369. https://doi.org/10.1111/febs.15327.
- 32.Byersdorfer,A. The role of Fatty Acid oxidation in the metabolic reprograming of activated t-cells. Front. Immunol. 2014, 5, 641. https://doi.org/10.3389/fimmu.2014.00641.
- 33.DeBerardinis,J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The Biology of Cancer: Metabolic Reprogramming Fuels Cell Growth and Proliferation. Cell Metab. 2008, 7, 11–20. https://doi.org/10.1016/j.cmet.2007.10.002.
- 34.Desdín-Micó,; Soto-Heredero, G.; Mittelbrunn, M. Mitochondrial activity in T cells. Mitochondrion 2018, 41, 51–57. https://doi.org/10.1016/j.mito.2017.10.006.
- 35.Williams,C.; O’Neill, L.A.J. A Role for the Krebs Cycle Intermediate Citrate in Metabolic Reprogramming in Innate Immunity and Inflammation. Front. Immunol. 2018, 9, 141. https://doi.org/10.3389/fimmu.2018.00141.
- 36.Cruzat,; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. https://doi.org/10.3390/nu10111564.
- 37.Yu,; Wang, Z.; Zhang, K.; Chi, Z.; Xu, T.; Jiang, D.; Chen, S.; Li, W.; Yang, X.; Zhang, X.; et al. One-Carbon Metabolism Supports S-Adenosylmethionine and Histone Methylation to Drive Inflammatory Macrophages. Mol. Cell 2019, 75, 1147–1160.e1145. https://doi.org/10.1016/j.molcel.2019.06.039.
- 38.Wu,; Yang, M.; Gaur, U.; Xu, H.; Yao, Y.; Li, D. Alpha-Ketoglutarate: Physiological Functions and Applications. Biomol. Ther. 2016, 24, 1–8. https://doi.org/10.4062/biomolther.2015.078.
- 39.Zhang,; Sheng, Q.; Zhao, N.; Huang, S.; Zhao, Y. DNA hypomethylation mediates immune response in pan-cancer. Epigenetics 2023, 18, 2192894. https://doi.org/10.1080/15592294.2023.2192894.
- 40.Renaudineau,; Ballestar, E. Epigenetics: DNA methylation signatures in Sjögren syndrome. Nat. Rev. Rheumatol. 2016, 12, 565–566. https://doi.org/10.1038/nrrheum.2016.144.
- 41.Miceli-Richard,; Wang-Renault, S.F.; Boudaoud, S.; Busato, F.; Lallemand, C.; Bethune, K.; Belkhir, R.; Nocturne, G.; Mariette, X.; Tost, J. Overlap between differentially methylated DNA regions in blood B lymphocytes and genetic at-risk loci in primary Sjögren’s syndrome. Ann. Rheum. Dis. 2016, 75, 933–940. https://doi.org/10.1136/annrheumdis-2014-206998.
- 42.Wang,; Riaz, F.; Wang, W.; Pu, J.; Liang, Y.; Wu, Z.; Pan, S.; Song, J.; Yang, L.; Zhang, Y.; et al. Functional significance of DNA methylation: Epigenetic insights into Sjogren’s syndrome. Front. Immunol. 2024, 15, 1289492. https://doi.org/10.3389/fimmu.2024.1289492.
- 43.Wieczorek,; Bigaud, M.; Pfister, S.; Ceci, M.; McMichael, K.; Afatsawo, C.; Hamburger, M.; Texier, C.; Henry, M.; Cojean, C.; et al. Blockade of CD40-CD154 pathway interactions suppresses ectopic lymphoid structures and inhibits pathology in the NOD/ShiLtJ mouse model of Sjögren’s syndrome. Ann. Rheum. Dis. 2019, 78, 974–978. https://doi.org/10.1136/annrheumdis-2018-213929.
- 44.Fisher,A.; Szanto, A.; Ng, W.F.; Bombardieri, M.; Posch, M.G.; Papas, A.S.; Farag, A.M.; Daikeler, T.; Bannert, B.; Kyburz, D.; et al. Assessment of the anti-CD40 antibody iscalimab in patients with primary Sjögren’s syndrome: A multicentre, randomised, double-blind, placebo-controlled, proof-of-concept study. Lancet Rheumatol. 2020, 2, e142–e152. https://doi.org/10.1016/s2665-9913(19)30135-3.
- 45.Saraux,; Pers, J.O.; Devauchelle-Pensec, V. Treatment of primary Sjögren syndrome. Nat. Rev. Rheumatol. 2016, 12, 456–471. https://doi.org/10.1038/nrrheum.2016.100.
- 46.Mathews,A.; Kurien, B.T.; Scofield, R.H. Oral manifestations of Sjögren’s syndrome. J. Dent. Res. 2008, 87, 308–318. https://doi.org/10.1177/154405910808700411.
- 47.Fana,; Dohn, U.M.; Krabbe, S.; Terslev, L. Application of the OMERACT Grey-scale Ultrasound Scoring System for salivary glands in a single-centre cohort of patients with suspected Sjögren’s syndrome. RMD Open 2021, 7, e001516. https://doi.org/10.1136/rmdopen-2020-001516.
- 48.Ramos-Casals,; Brito-Zerón, P.; Bombardieri, S.; Bootsma, H.; De Vita, S.; Dörner, T.; Fisher, B.A.; Gottenberg, J.E.; Hernandez-Molina, G.; Kocher, A.; et al. EULAR recommendations for the management of Sjögren’s syndrome with topical and systemic therapies. Ann. Rheum. Dis. 2020, 79, 3–18. https://doi.org/10.1136/annrheumdis-2019-216114.
- 49.Misuno,; Tran, S.D.; Khalili, S.; Huang, J.; Liu, Y.; Hu, S. Quantitative analysis of protein and gene expression in salivary glands of Sjogren’s-like disease NOD mice treated by bone marrow soup. PLoS ONE 2014, 9, e87158. https://doi.org/10.1371/journal.pone.0087158.
- 50.Tran,D.; Liu, Y.; Xia, D.; Maria, O.M.; Khalili, S.; Wang, R.W.; Quan, V.H.; Hu, S.; Seuntjens, J. Paracrine effects of bone marrow soup restore organ function, regeneration, and repair in salivary glands damaged by irradiation. PLoS ONE 2013, 8, e61632. https://doi.org/10.1371/journal.pone.0061632.
- 51.Baldini,; Fulvio, G.; La Rocca, G.; Ferro, F. Update on the pathophysiology and treatment of primary Sjögren syndrome. Nat. Rev. Rheumatol. 2024, 20, 473–491. https://doi.org/10.1038/s41584-024-01135-3.
- 52.Seror,; Nocturne, G.; Mariette, X. Current and future therapies for primary Sjögren syndrome. Nat. Rev. Rheumatol. 2021, 17, 475–486. https://doi.org/10.1038/s41584-021-00634-x.
How to Cite
Tang, D. C.; Hu, S. Pathogenic Mechanisms and Treatment Advancements of Sjogren’s Syndrome. Health and Metabolism 2025, 2 (2), 8. https://doi.org/10.53941/hm.2025.100015.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


