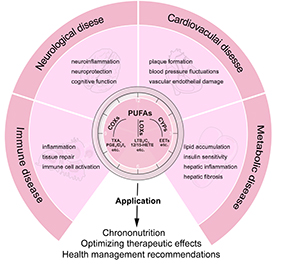
Polyunsaturated fatty acids (PUFAs) are essential lipid components that maintain human health and take part in various physiological and pathological processes. PUFAs are metabolized to bioactive mediators, such as prostaglandins (PGs), leukotrienes (LTs), epoxyeicosatrienoic acids (EETs), and hydroxyeicosatrienoic acids (HETEs), which play critical roles in cardiovascular function, metabolic homeostasis, neural activity, and inflammatory responses. Emerging evidence has shown that the circadian clock regulates the metabolism of PUFAs, exhibiting marked circadian rhythms varying across different disease states. This review explores the circadian dynamics of PUFAs metabolism and its implications in cardiovascular disease, metabolic disorders, neurodegenerative conditions, and immune diseases. Special attention is given to the circadian expression changes in PUFAs metabolic enzymes, like cyclooxygenases (COXs), lipoxygenases (LOXs), and cytochrome P450s (CYPs), and their potential mechanisms in disease development. In addition, the review discusses the application of circadian rhythms of PUFAs metabolism to optimize clinical strategies such as chronotherapy and personalized medicine. Understanding the circadian regulation in PUFAs metabolism could unveil new insights into disease mechanisms and inspire innovative approaches for the prevention and treatment of multiple diseases.
- Open Access
- Review
Physiological and Pathological Insights into the Circadian Rhythm of Polyunsaturated Fatty Acids Metabolism
- Ping Dai 1, 2,
- Jun-Yan Liu 1, 2, 3, 4, *
Author Information
Received: 05 Dec 2024 | Revised: 26 Dec 2024 | Accepted: 01 Apr 2025 | Published: 07 Jul 2025
Abstract
Graphical Abstract
Keywords
polyunsaturated fatty acids (PUFAs) | cyclooxygenases (COXs) | lipoxygenases (LOXs) | cytochrome P450s (CYPs) | circadian rhythm
References
- 1.Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. https://doi.org/10.1016/s0753-3322(02)00253-6.
- 2.Calder, P.C. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 197–202.
- 3.Meng, Y.W.; Liu, J.Y. Pathological and pharmacological functions of the metabolites of polyunsaturated sfatty acids mediated by cyclooxygenases, lipoxygenases, and cytochrome P450s in cancers. Pharmacol. Ther. 2024, 256, 108612. https://doi.org/10.1016/j.pharmthera.2024.108612.
- 4.Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. https://doi.org/10.1038/s41392-020-00443-w.
- 5.Iñiguez, M.A.; Cacheiro-Llaguno, C.; Cuesta, N.; Díaz-Muñoz, M.D.; Fresno, M. Prostanoid function and cardiovascular disease. Arch. Physiol. Biochem. 2008, 114, 201–209. https://doi.org/10.1080/13813450802180882.
- 6.McLennan, P.L.; Owen, A.J.; Slee, E.L.; Theiss, M.L. Myocardial function, ischaemia and n-3 polyunsaturated fatty acids: A membrane basis. J. Cardiovasc. Med. 2007, 8, S15–S18. https://doi.org/10.2459/01.JCM.0000289272.87803.ce.
- 7.Serhan, C.N.; Brain, S.D.; Buckley, C.D.; Gilroy, D.W.; Haslett, C.; O’Neill, L.A.; Perretti, M.; Rossi, A.G.; Wallace, J.L. Resolution of inflammation: State of the art, definitions and terms. Faseb J. 2007, 21, 325–332. https://doi.org/10.1096/fj.06-7227rev.
- 8.Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Health implications of high dietary omega-6 polyunsaturated Fatty acids. J. Nutr. Metab. 2012, 2012, 539426. https://doi.org/10.1155/2012/539426.
- 9.Lian, M.; Luo, W.; Sui, Y.; Li, Z.; Hua, J. Dietary n-3 PUFA Protects Mice from Con A Induced Liver Injury by Modulating Regulatory T Cells and PPAR-γ Expression. PLoS ONE 2015, 10, e0132741. https://doi.org/10.1371/journal.pone.0132741.
- 10.Panda, S. Circadian physiology of metabolism. Science 2016, 354, 1008–1015. https://doi.org/10.1126/science.aah4967.
- 11.Panda, S.; Hogenesch, J.B.; Kay, S.A. Circadian rhythms from flies to human. Nature 2002, 417, 329–335. https://doi.org/10.1038/417329a.
- 12.Patke, A.; Young, M.W.; Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2019, 21, 67–84. https://doi.org/10.1038/s41580-019-0179-2.
- 13.Mills, J.N.; Minors, D.S.; Waterhouse, J.M. The circadian rhythms of human subjects without timepieces or indication of the alternation of day and night. J. Physiol. 1974, 240, 567–594. https://doi.org/10.1113/jphysiol.1974.sp010623.
- 14.Guan, D.; Lazar, M.A. Interconnections between circadian clocks and metabolism. J. Clin. Investig. 2021, 131. https://doi.org/10.1172/jci148278.
- 15.Gnocchi, D.; Pedrelli, M.; Hurt-Camejo, E.; Parini, P. Lipids around the Clock: Focus on Circadian Rhythms and Lipid Metabolism. Biology 2015, 4, 104–132. https://doi.org/10.3390/biology4010104.
- 16.Vatier, C.; Christin-Maitre, S. Epigenetic/circadian clocks and PCOS. Hum. Reprod. 2024, 39, 1167–1175. https://doi.org/10.1093/humrep/deae066.
- 17.Arble, D.M.; Ramsey, K.M.; Bass, J.; Turek, F.W. Circadian disruption and metabolic disease: Findings from animal models. Best. Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 785–800. https://doi.org/10.1016/j.beem.2010.08.003.
- 18.Gabryelska, A.; Turkiewicz, S.; Karuga, F.F.; Sochal, M.; Strzelecki, D.; Białasiewicz, P. Disruption of Circadian Rhythm Genes in Obstructive Sleep Apnea Patients-Possible Mechanisms Involved and Clinical Implication. Int. J. Mol. Sci. 2022, 23, 709. https://doi.org/10.3390/ijms23020709.
- 19.Martemucci, G.; Khalil, M.; Di Luca, A.; Abdallah, H.; D’Alessandro, A.G. Comprehensive Strategies for Metabolic Syndrome: How Nutrition, Dietary Polyphenols, Physical Activity, and Lifestyle Modifications Address Diabesity, Cardiovascular Diseases, and Neurodegenerative Conditions. Metabolites 2024, 14, 327. https://doi.org/10.3390/metabo14060327.
- 20.Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. https://doi.org/10.3181/0711-mr-311.
- 21.Gupta, R.; Lakshmy, R.; Abraham, R.A.; Reddy, K.S.; Jeemon, P.; Prabhakaran, D. Serum Omega-6/Omega-3 Ratio and Risk Markers for Cardiovascular Disease in an Industrial Population of Delhi. Food Nutr. Sci. 2013, 4, 94–97. https://doi.org/10.4236/fns.2013.49A1015.
- 22.Robinson, L.E.; Buchholz, A.C.; Mazurak, V.C. Inflammation, obesity, and fatty acid metabolism: Influence of n-3 polyunsaturated fatty acids on factors contributing to metabolic syndrome. Appl. Physiol. Nutr. Metab. 2007, 32, 1008–1024. https://doi.org/10.1139/h07-087.
- 23.Negi, P.C.; Sharma, C.K.; Nihjawan, R.; Sharma, R.; Asotra, S. Role of omega 3 and omega 6 poly unsaturated fatty acids (PUFA) and vitamin D deficiency as risk determinants of metabolic syndrome in obesity: Worksite based case-control observational study. Diabetes Metab. Syndr. 2022, 16, 102467. https://doi.org/10.1016/j.dsx.2022.102467.
- 24.Khalili, L.; Valdes-Ramos, R.; Harbige, L.S. Effect of n-3 (Omega-3) Polyunsaturated Fatty Acid Supplementation on Metabolic and Inflammatory Biomarkers and Body Weight in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of RCTs. Metabolites 2021, 11, 742. https://doi.org/10.3390/metabo11110742.
- 25.Farooqui, A.A.; Farooqui, T.; Panza, F.; Frisardi, V. Metabolic syndrome as a risk factor for neurological disorders. Cell Mol. Life Sci. 2012, 69, 741–762. https://doi.org/10.1007/s00018-011-0840-1.
- 26.Rojas-Gutierrez, E.; Muñoz-Arenas, G.; Treviño, S.; Espinosa, B.; Chavez, R.; Rojas, K.; Flores, G.; Díaz, A.; Guevara, J. Alzheimer’s disease and metabolic syndrome: A link from oxidative stress and inflammation to neurodegeneration. Synapse 2017, 71, e21990. https://doi.org/10.1002/syn.21990.
- 27.Lubrano, V.; Ndreu, R.; Balzan, S. Classes of Lipid Mediators and Their Effects on Vascular Inflammation in Atherosclerosis. Int. J. Mol. Sci. 2023, 24, 1637. https://doi.org/10.3390/ijms24021637.
- 28.Panezai, J.; Van Dyke, T.E. Resolution of inflammation: Intervention strategies and future applications. Toxicol. Appl. Pharmacol. 2022, 449, 116089. https://doi.org/10.1016/j.taap.2022.116089.
- 29.Margină, D.; Ungurianu, A.; Purdel, C.; Nițulescu, G.M.; Tsoukalas, D.; Sarandi, E.; Thanasoula, M.; Burykina, T.I.; Tekos, F.; Buha, A.; et al. Analysis of the intricate effects of polyunsaturated fatty acids and polyphenols on inflammatory pathways in health and disease. Food Chem. Toxicol. 2020, 143, 111558. https://doi.org/10.1016/j.fct.2020.111558.
- 30.Tucker, K.L. Assessment of usual dietary intake in population studies of gene-diet interaction. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 74–81. https://doi.org/10.1016/j.numecd.2006.07.010.
- 31.Bass, J.; Takahashi, J.S. Circadian integration of metabolism and energetics. Science 2010, 330, 1349–1354. https://doi.org/10.1126/science.1195027.
- 32.Kulesza, A.; Paczek, L.; Burdzinska, A. The Role of COX-2 and PGE2 in the Regulation of Immunomodulation and Other Functions of Mesenchymal Stromal Cells. Biomedicines 2023, 11, 445. https://doi.org/10.3390/biomedicines11020445.
- 33.Ribas-Latre, A.; Eckel-Mahan, K. Nutrients and the Circadian Clock: A Partnership Controlling Adipose Tissue Function and Health. Nutrients 2022, 14, 2084. https://doi.org/10.3390/nu14102084.
- 34.Curtis, A.M.; Cheng, Y.; Kapoor, S.; Reilly, D.; Price, T.S.; Fitzgerald, G.A. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc. Natl. Acad. Sci. USA 2007, 104, 3450–3455. https://doi.org/10.1073/pnas.0611680104.
- 35.Hayashi, M.; Shimba, S.; Tezuka, M. Characterization of the molecular clock in mouse peritoneal macrophages. Biol. Pharm. Bull. 2007, 30, 621–626. https://doi.org/10.1248/bpb.30.621.
- 36.Prasai, M.J.; Mughal, R.S.; Wheatcroft, S.B.; Kearney, M.T.; Grant, P.J.; Scott, E.M. Diurnal variation in vascular and metabolic function in diet-induced obesity: Divergence of insulin resistance and loss of clock rhythm. Diabetes 2013, 62, 1981–1989. https://doi.org/10.2337/db11-1740.
- 37.Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. https://doi.org/10.3390/antiox12020517.
- 38.Ingle, K.A.; Kain, V.; Goel, M.; Prabhu, S.D.; Young, M.E.; Halade, G.V. Cardiomyocyte-specific Bmal1 deletion in mice triggers diastolic dysfunction, extracellular matrix response, and impaired resolution of inflammation. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1827–H1836. https://doi.org/10.1152/ajpheart.00608.2015.
- 39.Keller, M.; Mazuch, J.; Abraham, U.; Eom, G.D.; Herzog, E.D.; Volk, H.D.; Kramer, A.; Maier, B. A circadian clock in macrophages controls inflammatory immune responses. Proc. Natl. Acad. Sci. USA 2009, 106, 21407–21412. https://doi.org/10.1073/pnas.0906361106.
- 40.Reina-Couto, M.; Vale, L.; Carvalho, J.; Bettencourt, P.; Albino-Teixeira, A.; Sousa, T. Resolving Inflammation in Heart Failure: Novel Protective Lipid Mediators. Curr. Drug Targets 2016, 17, 1206–1223. https://doi.org/10.2174/1389450117666160101121135.
- 41.Cutolo, M. SP0040 The Immune/Inflammatory Response Follows Circadian Rhythms. Ann. Rheum. Dis. 2014, 73, 11–11.
- 42.Zielinski, M.R.; Gibbons, A.J. Neuroinflammation, sleep, and circadian rhythms. Front. Cell. Infect. Microbiol. 2022, 12, 853096.
- 43.Spies, C.M.; Cutolo, M.; Straub, R.H.; Burmester, G.-R.; Buttgereit, F. More night than day—circadian rhythms in polymyalgia rheumatica and ankylosing spondylitis. J. Rheumatol. 2010, 37, 894–899.
- 44.Shirato, K.; Sato, S. Macrophage meets the circadian clock: Implication of the circadian clock in the role of macrophages in acute lower respiratory tract infection. Front. Cell. Infect. Microbiol. 2022, 12, 826738.
- 45.Gooley, J.J. Circadian regulation of lipid metabolism. Proc. Nutr. Soc. 2016, 75, 440–450. https://doi.org/10.1017/s0029665116000288.
- 46.Okyar, A.; Ozturk Civelek, D.; Akyel, Y.K.; Surme, S.; Pala Kara, Z.; Kavakli, I.H. The role of the circadian timing system on drug metabolism and detoxification: An update. Expert. Opin. Drug Metab. Toxicol. 2024, 20, 503–517. https://doi.org/10.1080/17425255.2024.2356167.
- 47.Skowronek, R.; Czekaj, P.; Suszka-Świtek, A.; Czech, E.; Wiaderkiewicz, A.; Plewka, D.; Bryzek, A. Expression of cytochrome P450 2C and 3A in female rat liver after long-term administration of gonadoliberin analogs. Int. J. Occup. Med. Environ. Health 2016, 29, 293–314. https://doi.org/10.13075/ijomeh.1896.00528.
- 48.Carver, K.A.; Lourim, D.; Tryba, A.K.; Harder, D.R. Rhythmic expression of cytochrome P450 epoxygenases CYP4x1 and CYP2c11 in the rat brain and vasculature. Am. J. Physiol. Cell Physiol. 2014, 307, C989–C998. https://doi.org/10.1152/ajpcell.00401.2013.
- 49.Zhu, X.; Hou, Q.; Zhang, L.; Wang, D.; Tian, Z.; Liu, Y.; Wang, Y.; Li, Y.; Jiang, H. Isorhynchophylline improves lipid metabolism disorder by mediating a circadian rhythm gene Bmal1 in spontaneously hypertensive rat. Phytother. Res. 2023, 37, 5991–6005. https://doi.org/10.1002/ptr.8015.
- 50.Zardoya, R.; Diez, A.; Serradilla, M.C.; Madrid, J.A.; Bautista, J.M.; Garrido-Pertierra, A. Lipogenic activities in rat liver are subjected to circadian rhythms. Rev. Esp. Fisiol. 1994, 50, 239–244.
- 51.Ma, D.; Liu, T.; Chang, L.; Rui, C.; Xiao, Y.; Li, S.; Hogenesch, J.B.; Chen, Y.E.; Lin, J.D. The Liver Clock Controls Cholesterol Homeostasis through Trib1 Protein-mediated Regulation of PCSK9/Low Density Lipoprotein Receptor (LDLR) Axis. J. Biol. Chem. 2015, 290, 31003–31012. https://doi.org/10.1074/jbc.M115.685982.
- 52.Hussain, M.M.; Pan, X. Circadian regulators of intestinal lipid absorption. J. Lipid Res. 2015, 56, 761–770. https://doi.org/10.1194/jlr.R051573.
- 53.Poggiogalle, E.; Jamshed, H.; Peterson, C.M. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism 2018, 84, 11–27. https://doi.org/10.1016/j.metabol.2017.11.017.
- 54.Das, U.N. “Cell Membrane Theory of Senescence” and the Role of Bioactive Lipids in Aging, and Aging Associated Diseases and Their Therapeutic Implications. Biomolecules 2021, 11, 241. https://doi.org/10.3390/biom11020241.
- 55.Fagiani, F.; Di Marino, D.; Romagnoli, A.; Travelli, C.; Voltan, D.; Di Cesare Mannelli, L.; Racchi, M.; Govoni, S.; Lanni, C. Molecular regulations of circadian rhythm and implications for physiology and diseases. Signal Transduct. Target. Ther. 2022, 7, 41. https://doi.org/10.1038/s41392-022-00899-y.
- 56.Xu, H.; Li, H.; Woo, S.L.; Kim, S.M.; Shende, V.R.; Neuendorff, N.; Guo, X.; Guo, T.; Qi, T.; Pei, Y.; et al. Myeloid cell-specific disruption of Period1 and Period2 exacerbates diet-induced inflammation and insulin resistance. J. Biol. Chem. 2014, 289, 16374–16388. https://doi.org/10.1074/jbc.M113.539601.
- 57.Downton, P.; Sanna, F.; Maidstone, R.; Poolman, T.M.; Hayter, E.A.; Dickson, S.H.; Ciccone, N.A.; Early, J.O.; Adamson, A.; Spiller, D.G.; et al. Chronic inflammatory arthritis drives systemic changes in circadian energy metabolism. Proc. Natl. Acad. Sci. USA 2022, 119, e2112781119. https://doi.org/10.1073/pnas.2112781119.
- 58.Bellet, M.M.; Masri, S.; Astarita, G.; Sassone-Corsi, P.; Della Fazia, M.A.; Servillo, G. Histone Deacetylase SIRT1 Controls Proliferation, Circadian Rhythm, and Lipid Metabolism during Liver Regeneration in Mice. J. Biol. Chem. 2016, 291, 23318–23329. https://doi.org/10.1074/jbc.M116.737114.
- 59.Shen, W.; Wang, C.; Xia, L.; Fan, C.; Dong, H.; Deckelbaum, R.J.; Qi, K. Epigenetic modification of the leptin promoter in diet-induced obese mice and the effects of N-3 polyunsaturated fatty acids. Sci. Rep. 2014, 4, 5282. https://doi.org/10.1038/srep05282.
- 60.Zhao, X.; Zhu, X.; Cheng, S.; Xie, Y.; Wang, Z.; Liu, Y.; Jiang, Z.; Xiao, J.; Guo, H.; Wang, Y. MiR-29a/b/c regulate human circadian gene hPER1 expression by targeting its 3’UTR. Acta Biochim. Biophys. Sin. 2014, 46, 313–317. https://doi.org/10.1093/abbs/gmu007.
- 61.Shende, V.R.; Goldrick, M.M.; Ramani, S.; Earnest, D.J. Expression and rhythmic modulation of circulating microRNAs targeting the clock gene Bmal1 in mice. PLoS ONE 2011, 6, e22586. https://doi.org/10.1371/journal.pone.0022586.
- 62.Milagro, F.I.; Gómez-Abellán, P.; Campión, J.; Martínez, J.A.; Ordovás, J.M.; Garaulet, M. CLOCK, PER2 and BMAL1 DNA methylation: Association with obesity and metabolic syndrome characteristics and monounsaturated fat intake. Chronobiol. Int. 2012, 29, 1180–1194. https://doi.org/10.3109/07420528.2012.719967.
- 63.Shende, V.R.; Neuendorff, N.; Earnest, D.J. Role of miR-142-3p in the post-transcriptional regulation of the clock gene Bmal1 in the mouse SCN. PLoS ONE 2013, 8, e65300. https://doi.org/10.1371/journal.pone.0065300.
- 64.Garaulet, M.; Lee, Y.-C.; Shen, J.; Parnell, L.D.; Arnett, D.K.; Tsai, M.Y.; Lai, C.-Q.; Ordovas, J.M. CLOCK genetic variation and metabolic syndrome risk: Modulation by monounsaturated fatty acids. Am. J. Clin. Nutr. 2009, 90, 1466–1475.
- 65.Kissling, C.; Retz, W.; Wiemann, S.; Coogan, A.N.; Clement, R.M.; Hünnerkopf, R.; Conner, A.C.; Freitag, C.M.; Rösler, M.; Thome, J. A polymorphism at the 3’-untranslated region of the CLOCK gene is associated with adult attention-deficit hyperactivity disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008, 147, 333–338. https://doi.org/10.1002/ajmg.b.30602.
- 66.Camblor Murube, M.; Borregon-Rivilla, E.; Colmenarejo, G.; Aguilar-Aguilar, E.; Martínez, J.A.; Ramírez De Molina, A.; Reglero, G.; Loria-Kohen, V. Polymorphism of CLOCK Gene rs3749474 as a Modulator of the Circadian Evening Carbohydrate Intake Impact on Nutritional Status in an Adult Sample. Nutrients 2020, 12, 1142. https://doi.org/10.3390/nu12041142.
- 67.Maury, E.; Hong, H.K.; Bass, J. Circadian disruption in the pathogenesis of metabolic syndrome. Diabetes Metab. 2014, 40, 338–346. https://doi.org/10.1016/j.diabet.2013.12.005.
- 68.Leu, H.B.; Chung, C.M.; Lin, S.J.; Chiang, K.M.; Yang, H.C.; Ho, H.Y.; Ting, C.T.; Lin, T.H.; Sheu, S.H.; Tsai, W.C.; et al. Association of circadian genes with diurnal blood pressure changes and non-dipper essential hypertension: a genetic association with young-onset hypertension. Hypertens. Res. 2015, 38, 155–162. https://doi.org/10.1038/hr.2014.152.
- 69.Yamaguchi, M.; Uemura, H.; Arisawa, K.; Katsuura-Kamano, S.; Hamajima, N.; Hishida, A.; Suma, S.; Oze, I.; Nakamura, K.; Takashima, N.; et al. Association between brain-muscle-ARNT-like protein-2 (BMAL2) gene polymorphism and type 2 diabetes mellitus in obese Japanese individuals: A cross-sectional analysis of the Japan Multi-institutional Collaborative Cohort Study. Diabetes Res. Clin. Pract. 2015, 110, 301–308. https://doi.org/10.1016/j.diabres.2015.10.009.
- 70.Nascimento Ferreira, M.V.; Goumidi, L.; Carvalho, H.B.; De Moraes, A.C.F.; Santaliestra-Pasías, A.M.; Kafatos, A.; Molnar, D.; Lambrinou, C.P.; De Henauw, S.; Gutierrez, A.; et al. Associations between REV-ERBα, sleep duration and body mass index in European adolescents. Sleep. Med. 2018, 46, 56–60. https://doi.org/10.1016/j.sleep.2018.01.014.
- 71.Lin, Y.; He, L.; Cai, Y.; Wang, X.; Wang, S.; Li, F. The role of circadian clock in regulating cell functions: Implications for diseases. MedComm 2024, 5, e504. https://doi.org/10.1002/mco2.504.
- 72.D’Angelo, S.; Motti, M.L.; Meccariello, R. ω-3 and ω-6 Polyunsaturated Fatty Acids, Obesity and Cancer. Nutrients 2020, 12, 2751. https://doi.org/10.3390/nu12092751.
- 73.Namgyal, D.; Chandan, K.; Sultan, A.; Aftab, M.; Ali, S.; Mehta, R.; El-Serehy, H.A.; Al-Misned, F.A.; Sarwat, M. Dim Light at Night Induced Neurodegeneration and Ameliorative Effect of Curcumin. Cells 2020, 9, 2093. https://doi.org/10.3390/cells9092093.
- 74.Mora, I.; Arola, L.; Caimari, A.; Escoté, X.; Puiggròs, F. Structured Long-Chain Omega-3 Fatty Acids for Improvement of Cognitive Function during Aging. Int. J. Mol. Sci. 2022, 23, 3472. https://doi.org/10.3390/ijms23073472.
- 75.Checa-Ros, A.; D’Marco, L. Role of Omega-3 Fatty Acids as Non-Photic Zeitgebers and Circadian Clock Synchronizers. Int. J. Mol. Sci. 2022, 23, 12162. https://doi.org/10.3390/ijms232012162.
- 76.Tsuchiya, Y.; Minami, I.; Kadotani, H.; Nishida, E. Resetting of peripheral circadian clock by prostaglandin E2. EMBO Rep. 2005, 6, 256–261. https://doi.org/10.1038/sj.embor.7400356.
- 77.Chen, S.; Fuller, K.K.; Dunlap, J.C.; Loros, J.J. A Pro- and Anti-inflammatory Axis Modulates the Macrophage Circadian Clock. Front. Immunol. 2020, 11, 867. https://doi.org/10.3389/fimmu.2020.00867.
- 78.Baumann, A.; Gönnenwein, S.; Bischoff, S.C.; Sherman, H.; Chapnik, N.; Froy, O.; Lorentz, A. The circadian clock is functional in eosinophils and mast cells. Immunology 2013, 140, 465–474. https://doi.org/10.1111/imm.12157.
- 79.Wang, Y.; Pati, P.; Xu, Y.; Chen, F.; Stepp, D.W.; Huo, Y.; Rudic, R.D.; Fulton, D.J. Endotoxin Disrupts Circadian Rhythms in Macrophages via Reactive Oxygen Species. PLoS ONE 2016, 11, e0155075. https://doi.org/10.1371/journal.pone.0155075.
- 80.Baxter, M.; Ray, D.W. Circadian rhythms in innate immunity and stress responses. Immunology 2020, 161, 261–267. https://doi.org/10.1111/imm.13166.
- 81.Wang, Q.; Maillard, M.; Schibler, U.; Burnier, M.; Gachon, F. Cardiac hypertrophy, low blood pressure, and low aldosterone levels in mice devoid of the three circadian PAR bZip transcription factors DBP, HLF, and TEF. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1013–R1019. https://doi.org/10.1152/ajpregu.00241.2010.
- 82.Man, A.W.C.; Li, H.; Xia, N. Circadian Rhythm: Potential Therapeutic Target for Atherosclerosis and Thrombosis. Int. J. Mol. Sci. 2021, 22, 676. https://doi.org/10.3390/ijms22020676.
- 83.Naito, Y.; Tsujino, T.; Kawasaki, D.; Okumura, T.; Morimoto, S.; Masai, M.; Sakoda, T.; Fujioka, Y.; Ohyanagi, M.; Iwasaki, T. Circadian gene expression of clock genes and plasminogen activator inhibitor-1 in heart and aorta of spontaneously hypertensive and Wistar-Kyoto rats. J. Hypertens. 2003, 21, 1107–1115. https://doi.org/10.1097/00004872-200306000-00010.
- 84.Smyth, E.M. Thromboxane and the thromboxane receptor in cardiovascular disease. Clin. Lipidol. 2010, 5, 209.
- 85.Félétou, M.; Huang, Y.; Vanhoutte, P.M. Endothelium-mediated control of vascular tone: COX-1 and COX-2 products. Br. J. Pharmacol. 2011, 164, 894–912.
- 86.Liu, D.; Ji, L.; Wang, Y.; Zheng, L. Cyclooxygenase-2 expression, prostacyclin production and endothelial protection of high-density lipoprotein. Cardiovasc. Hematol. Disord. Drug Targets 2012, 12, 98–105. https://doi.org/10.2174/1871529x11202020098.
- 87.Chen, H. Role of thromboxane A2 signaling in endothelium-dependent contractions of arteries. Prostaglandins Other Lipid Mediat. 2018, 134, 32–37.
- 88.Wong, M.S.-K.; Vanhoutte, P.M. COX-mediated endothelium-dependent contractions: From the past to recent discoveries. Acta Pharmacol. Sin. 2010, 31, 1095–1102.
- 89.Hristovska, A.-M.; Rasmussen, L.E.; Hansen, P.B.; Nielsen, S.S.; Nüsing, R.M.; Narumiya, S.; Vanhoutte, P.; Skøtt, O.; Jensen, B.L. Prostaglandin E2 induces vascular relaxation by E-prostanoid 4 receptor-mediated activation of endothelial nitric oxide synthase. Hypertension 2007, 50, 525–530.
- 90.Eskildsen, M.P.; Hansen, P.B.; Stubbe, J.; Toft, A.; Walter, S.; Marcussen, N.; Rasmussen, L.M.; Vanhoutte, P.M.; Jensen, B.L. Prostaglandin I2 and prostaglandin E2 modulate human intrarenal artery contractility through prostaglandin E2-EP4, prostacyclin-IP, and thromboxane A2-TP receptors. Hypertension 2014, 64, 551–556.
- 91.Lang, P.; Kempe, D.; Myssina, S.; Tanneur, V.; Birka, C.; Laufer, S.; Lang, F.; Wieder, T.; Huber, S. PGE2 in the regulation of programmed erythrocyte death. Cell Death Differ. 2005, 12, 415–428.
- 92.Cipollone, F.; Prontera, C.; Pini, B.; Marini, M.; Fazia, M.; De Cesare, D.; Iezzi, A.; Ucchino, S.; Boccoli, G.; Saba, V. Overexpression of functionally coupled cyclooxygenase-2 and prostaglandin E synthase in symptomatic atherosclerotic plaques as a basis of prostaglandin E2-dependent plaque instability. Circulation 2001, 104, 921–927.
- 93.Yang, G.; Chen, L. An update of microsomal prostaglandin E synthase-1 and PGE2 receptors in cardiovascular health and diseases. Oxidative Med. Cell. Longev. 2016, 2016, 5249086.
- 94.Caligiuri, S.P.B.; Aukema, H.M.; Ravandi, A.; Lavallée, R.; Guzman, R.; Pierce, G.N. Specific plasma oxylipins increase the odds of cardiovascular and cerebrovascular events in patients with peripheral artery disease. Can. J. Physiol. Pharmacol. 2017, 95, 961–968. https://doi.org/10.1139/cjpp-2016-0615.
- 95.Libby, P.; Ridker, P.M.; Hansson, G.K. Inflammation in atherosclerosis: From pathophysiology to practice. J. Am. Coll. Cardiol. 2009, 54, 2129–2138. https://doi.org/10.1016/j.jacc.2009.09.009.
- 96.Pignone, M.; Alberts, M.J.; Colwell, J.A.; Cushman, M.; Inzucchi, S.E.; Mukherjee, D.; Rosenson, R.S.; Williams, C.D.; Wilson, P.W.; Kirkman, M.S. Aspirin for primary prevention of cardiovascular events in people with diabetes. J. Am. Coll. Cardiol. 2010, 55, 2878–2886. https://doi.org/10.1016/j.jacc.2010.04.003.
- 97.Yang, J.; Xu, Z.F.; Su, J.; Fan, S.F.; Wang, J.Y.; Ji, L.D.; Chen, X.M. [Research progress on the circadian clock regulation in cardiovascular system and association between circadian clock regulation and cardiovascular diseases]. Zhonghua Xin Xue Guan Bing Za Zhi 2020, 48, 610–615. https://doi.org/10.3760/cma.j.cn112148-20190725-00430.
- 98.Zhang, J.; Sun, R.; Jiang, T.; Yang, G.; Chen, L. Circadian Blood Pressure Rhythm in Cardiovascular and Renal Health and Disease. Biomolecules 2021, 11, 868. https://doi.org/10.3390/biom11060868.
- 99.Aliwarga, T.; Evangelista, E.A.; Sotoodehnia, N.; Lemaitre, R.N.; Totah, R.A. Regulation of CYP2J2 and EET levels in cardiac disease and diabetes. Int. J. Mol. Sci. 2018, 19, 1916.
- 100.Bhatnagar, A. Beating ischemia: A new feat of EETs? Am. Heart Assoc. 2004, 95, 443–445.
- 101.Imig, J.D. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol. Rev. 2012, 92, 101–130.
- 102.Thosar, S.S.; Berman, A.M.; Herzig, M.X.; McHill, A.W.; Bowles, N.P.; Swanson, C.M.; Clemons, N.A.; Butler, M.P.; Clemons, A.A.; Emens, J.S. Circadian rhythm of vascular function in midlife adults. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1203–1211.
- 103.Kezeli, T.; Gongadze, N.; Sukoyan, G.; Shikhashvili, M.; Chapichadze, Z.; Okujava, M.; Dolidze, N. Circadian variation in vasoconstriction and vasodilation mediators and baroreflex sensitivity in hypertensive rats. J. Circadian Rhythm. 2019, 17, 10.
- 104.Gross, G.J.; Hsu, A.; Falck, J.R.; Nithipatikom, K. Mechanisms by which epoxyeicosatrienoic acids (EETs) elicit cardioprotection in rat hearts. J. Mol. Cell Cardiol. 2007, 42, 687–691. https://doi.org/10.1016/j.yjmcc.2006.11.020.
- 105.Xie, Z.; Su, W.; Liu, S.; Zhao, G.; Esser, K.; Schroder, E.A.; Lefta, M.; Stauss, H.M.; Guo, Z.; Gong, M.C. Smooth-muscle BMAL1 participates in blood pressure circadian rhythm regulation. J. Clin. Investig. 2015, 125, 324–336.
- 106.Gianazza, E.; Brioschi, M.; Fernandez, A.M.; Banfi, C. Lipoxidation in cardiovascular diseases. Redox Biol. 2019, 23, 101119.
- 107.Sonnweber, T.; Pizzini, A.; Nairz, M.; Weiss, G.; Tancevski, I. Arachidonic acid metabolites in cardiovascular and metabolic diseases. Int. J. Mol. Sci. 2018, 19, 3285.
- 108.Bäck, M. Inflammatory signaling through leukotriene receptors in atherosclerosis. Curr. Atheroscler. Rep. 2008, 10, 244–251. https://doi.org/10.1007/s11883-008-0038-7.
- 109.Baker, N.; O’Meara, S.J.; Scannell, M.; Maderna, P.; Godson, C. Lipoxin A4: Anti-inflammatory and anti-angiogenic impact on endothelial cells. J. Immunol. 2009, 182, 3819–3826.
- 110.Kotlyarov, S.; Kotlyarova, A. Molecular pharmacology of inflammation resolution in atherosclerosis. Int. J. Mol. Sci. 2022, 23, 4808.
- 111.Pirault, J.; Bäck, M. Lipoxin and Resolvin Receptors Transducing the Resolution of Inflammation in Cardiovascular Disease. Front. Pharmacol. 2018, 9, 1273. https://doi.org/10.3389/fphar.2018.01273.
- 112.Sun, R.; Huang, J.; Yang, N.; He, J.; Yu, X.; Feng, S.; Xie, Y.; Wang, G.; Ye, H.; Aa, J. Purine Catabolism Shows a Dampened Circadian Rhythmicity in a High-fat Diet-Induced Mouse Model of Obesity. Molecules 2019, 24, 4524. https://doi.org/10.3390/molecules24244524.
- 113.Guesnet, P.; Alessandri, J.M.; Vancassel, S.; Zamaria, N. Analysis of the 2nd symposium “Anomalies of fatty acids, ageing and degenerating pathologies”. Reprod. Nutr. Dev. 2004, 44, 263–271. https://doi.org/10.1051/rnd:2004031.
- 114.Clarke, S.D.; Baillie, R.; Jump, D.B.; Nakamura, M.T. Fatty acid regulation of gene expression. Its role in fuel partitioning and insulin resistance. Ann. N. Y Acad. Sci. 1997, 827, 178–187. https://doi.org/10.1111/j.1749-6632.1997.tb51833.x.
- 115.Chan, P.C.; Liao, M.T.; Hsieh, P.S. The Dualistic Effect of COX-2-Mediated Signaling in Obesity and Insulin Resistance. Int. J. Mol. Sci. 2019, 20, 3115. https://doi.org/10.3390/ijms20133115.
- 116.Robertson, R.P. Dominance of cyclooxygenase-2 in the regulation of pancreatic islet prostaglandin synthesis. Diabetes 1998, 47, 1379–1383. https://doi.org/10.2337/diabetes.47.9.1379.
- 117.Cao, Y.; Mai, W.; Li, R.; Deng, S.; Li, L.; Zhou, Y.; Qin, Q.; Zhang, Y.; Zhou, X.; Han, M.; et al. Macrophages evoke autophagy of hepatic stellate cells to promote liver fibrosis in NAFLD mice via the PGE2/EP4 pathway. Cell Mol. Life Sci. 2022, 79, 303. https://doi.org/10.1007/s00018-022-04319-w.
- 118.Kaffe, E.; Tisi, A.; Magkrioti, C.; Aidinis, V.; Mehal, W.Z.; Flavell, R.A.; Maccarrone, M. Bioactive signalling lipids as drivers of chronic liver diseases. J. Hepatol. 2024, 80, 140–154. https://doi.org/10.1016/j.jhep.2023.08.029.
- 119.Nakao, T.; Yasumoto, A.; Tokuoka, S.; Kita, Y.; Kawahara, T.; Daimon, M.; Yatomi, Y. The impact of night-shift work on platelet function in healthy medical staff. J. Occup. Health 2018, 60, 324–332. https://doi.org/10.1539/joh.2018-0027-FS.
- 120.Radi, Z.A.; Khan, K.N. Cardio-renal safety of non-steroidal anti-inflammatory drugs. J. Toxicol. Sci. 2019, 44, 373–391. https://doi.org/10.2131/jts.44.373.
- 121.Gaiz, A.; Mosawy, S.; Colson, N.; Singh, I. Thrombotic and cardiovascular risks in type two diabetes; Role of platelet hyperactivity. Biomed. Pharmacother. 2017, 94, 679–686. https://doi.org/10.1016/j.biopha.2017.07.121.
- 122.Ma, K.; Jin, X.; Liang, X.; Zhao, Q.; Zhang, X. Inflammatory mediators involved in the progression of the metabolic syndrome. Diabetes Metab. Res. Rev. 2012, 28, 388–394. https://doi.org/10.1002/dmrr.2291.
- 123.Szczuko, M.; Kozioł, I.; Kotlęga, D.; Brodowski, J.; Drozd, A. The Role of Thromboxane in the Course and Treatment of Ischemic Stroke: Review. Int. J. Mol. Sci. 2021, 22, 11644. https://doi.org/10.3390/ijms222111644.
- 124.Dahik, V.D.; Frisdal, E.; Le Goff, W. Rewiring of Lipid Metabolism in Adipose Tissue Macrophages in Obesity: Impact on Insulin Resistance and Type 2 Diabetes. Int. J. Mol. Sci. 2020, 21, 5505. https://doi.org/10.3390/ijms21155505.
- 125.Khan, S.A.; Ali, A.; Khan, S.A.; Zahran, S.A.; Damanhouri, G.; Azhar, E.; Qadri, I. Unraveling the complex relationship triad between lipids, obesity, and inflammation. Mediators Inflamm. 2014, 2014, 502749. https://doi.org/10.1155/2014/502749.
- 126.Brennan, E.; Kantharidis, P.; Cooper, M.E.; Godson, C. Pro-resolving lipid mediators: Regulators of inflammation, metabolism and kidney function. Nat. Rev. Nephrol. 2021, 17, 725–739. https://doi.org/10.1038/s41581-021-00454-y.
- 127.Shi, D.; Chen, J.; Wang, J.; Yao, J.; Huang, Y.; Zhang, G.; Bao, Z. Circadian Clock Genes in the Metabolism of Non-alcoholic Fatty Liver Disease. Front. Physiol. 2019, 10, 423. https://doi.org/10.3389/fphys.2019.00423.
- 128.Chernyshev, O.Y.; McCarty, D.E.; Chesson, A.L. Inflammatory Mediators in Obstructive Sleep Apnea. In Neuroinflammation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 449–491.
- 129.Drozd, A.; Kotlęga, D.; Nowacki, P.; Ciećwież, S.; Trochanowski, T.; Szczuko, M. Fatty Acid Levels and Their Inflammatory Metabolites Are Associated with the Nondipping Status and Risk of Obstructive Sleep Apnea Syndrome in Stroke Patients. Biomedicines 2022, 10, 2200. https://doi.org/10.3390/biomedicines10092200.
- 130.Xu, X.; Li, R.; Chen, G.; Hoopes, S.L.; Zeldin, D.C.; Wang, D.W. The Role of Cytochrome P450 Epoxygenases, Soluble Epoxide Hydrolase, and Epoxyeicosatrienoic Acids in Metabolic Diseases. Adv. Nutr. 2016, 7, 1122–1128. https://doi.org/10.3945/an.116.012245.
- 131.Misheva, M.; Johnson, J.; McCullagh, J. Role of Oxylipins in the Inflammatory-Related Diseases NAFLD, Obesity, and Type 2 Diabetes. Metabolites 2022, 12, 1238. https://doi.org/10.3390/metabo12121238.
- 132.Pivovarova, O.; Jürchott, K.; Rudovich, N.; Hornemann, S.; Ye, L.; Möckel, S.; Murahovschi, V.; Kessler, K.; Seltmann, A.C.; Maser-Gluth, C.; et al. Changes of Dietary Fat and Carbohydrate Content Alter Central and Peripheral Clock in Humans. J. Clin. Endocrinol. Metab. 2015, 100, 2291–2302. https://doi.org/10.1210/jc.2014-3868.
- 133.Qian, J.; Yeh, B.; Rakshit, K.; Colwell, C.S.; Matveyenko, A.V. Circadian Disruption and Diet-Induced Obesity Synergize to Promote Development of β-Cell Failure and Diabetes in Male Rats. Endocrinology 2015, 156, 4426–4436. https://doi.org/10.1210/en.2015-1516.
- 134.Froy, O. Metabolism and circadian rhythms--implications for obesity. Endocr. Rev. 2010, 31, 1–24. https://doi.org/10.1210/er.2009-0014.
- 135.Lefebvre, P.; Chinetti, G.; Fruchart, J.C.; Staels, B. Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J. Clin. Investig. 2006, 116, 571–580. https://doi.org/10.1172/jci27989.
- 136.Salvati, S.; Attorri, L.; Di Benedetto, R.; Di Biase, A.; Leonardi, F. Polyunsaturated fatty acids and neurological diseases. Mini Rev. Med. Chem. 2006, 6, 1201–1211. https://doi.org/10.2174/138955706778742740.
- 137.Sheremeta, C.L.; Yarlagadda, S.; Smythe, M.L.; Noakes, P.G. Prostaglandins in the Inflamed Central Nervous System: Potential Therapeutic Targets. Curr. Drug Targets 2024, 25, 885–908. https://doi.org/10.2174/0113894501323980240815113851.
- 138.Vezzani, A.; Friedman, A.; Dingledine, R.J. The role of inflammation in epileptogenesis. Neuropharmacology 2013, 69, 16–24. https://doi.org/10.1016/j.neuropharm.2012.04.004.
- 139.Andreasson, K. Emerging roles of PGE2 receptors in models of neurological disease. Prostaglandins Other Lipid Mediat. 2010, 91, 104–112. https://doi.org/10.1016/j.prostaglandins.2009.04.003.
- 140.Gao, C.; Wang, H.; Wang, T.; Luo, C.; Wang, Z.; Zhang, M.; Chen, X.; Tao, L. Platelet regulates neuroinflammation and restores blood-brain barrier integrity in a mouse model of traumatic brain injury. J. Neurochem. 2020, 154, 190–204. https://doi.org/10.1111/jnc.14983.
- 141.Gorica, E.; Calderone, V. Arachidonic Acid Derivatives and Neuroinflammation. CNS Neurol. Disord. Drug Targets 2022, 21, 118–129. https://doi.org/10.2174/1871527320666210208130412.
- 142.Engblom, D.; Ek, M.; Saha, S.; Ericsson-Dahlstrand, A.; Jakobsson, P.J.; Blomqvist, A. Prostaglandins as inflammatory messengers across the blood-brain barrier. J. Mol. Med. 2002, 80, 5–15. https://doi.org/10.1007/s00109-001-0289-z.
- 143.Lu, J.; Xing, J.; Li, J. Prostaglandin E2 (PGE2) inhibits glutamatergic synaptic transmission in dorsolateral periaqueductal gray (dl-PAG). Brain Res. 2007, 1162, 38–47. https://doi.org/10.1016/j.brainres.2007.06.004.
- 144.Beura, S.K.; Dhapola, R.; Panigrahi, A.R.; Yadav, P.; Kumar, R.; Reddy, D.H.; Singh, S.K. Antiplatelet drugs: Potential therapeutic options for the management of neurodegenerative diseases. Med. Res. Rev. 2023, 43, 1835–1877. https://doi.org/10.1002/med.21965.
- 145.Murakami, M. Lipid mediators in life science. Exp. Anim. 2011, 60, 7–20. https://doi.org/10.1538/expanim.60.7.
- 146.Phillis, J.W.; Horrocks, L.A.; Farooqui, A.A. Cyclooxygenases, lipoxygenases, and epoxygenases in CNS: Their role and involvement in neurological disorders. Brain Res. Rev. 2006, 52, 201–243. https://doi.org/10.1016/j.brainresrev.2006.02.002.
- 147.Razavi, S.M.; Khayatan, D.; Arab, Z.N.; Momtaz, S.; Zare, K.; Jafari, R.M.; Dehpour, A.R.; Abdolghaffari, A.H. Licofelone, a potent COX/5-LOX inhibitor and a novel option for treatment of neurological disorders. Prostaglandins Other Lipid Mediat. 2021, 157, 106587. https://doi.org/10.1016/j.prostaglandins.2021.106587.
- 148.Farooqui, A.A.; Farooqui, A.A. Metabolism and Roles of Eicosanoids in Brain. In Lipid Mediators and Their Metabolism in the Brain; Springer: New York, NY, USA, 2011; pp. 1–47.
- 149.Farooqui, A.A. Lipid mediators and their metabolism in the nucleus: Implications for Alzheimer’s disease. Journal of Alzheimer’s Disease 2012, 30, S163–S178.
- 150.Kabata, H.; Moro, K.; Koyasu, S. The group 2 innate lymphoid cell (ILC2) regulatory network and its underlying mechanisms. Immunol. Rev. 2018, 286, 37–52. https://doi.org/10.1111/imr.12706.
- 151.Manev, H.; Uz, T.; Sugaya, K.; Qu, T. Putative role of neuronal 5-lipoxygenase in an aging brain. Faseb J. 2000, 14, 1464–1469. https://doi.org/10.1096/fj.14.10.1464.
- 152.Castro-Lopez, N.; Campuzano, A.; Mdalel, E.; Vanegas, D.; Chaturvedi, A.; Nguyen, P.; Pulse, M.; Cardona, A.E.; Wormley, F.L., Jr. Inhibition of host 5-lipoxygenase reduces overexuberant inflammatory responses and mortality associated with Cryptococcus meningoencephalitis. mBio 2024, 15, e0148324. https://doi.org/10.1128/mbio.01483-24.
- 153.Gonzalez-Fernandez, E.; Staursky, D.; Lucas, K.; Nguyen, B.V.; Li, M.; Liu, Y.; Washington, C.; Coolen, L.M.; Fan, F.; Roman, R.J. 20-HETE Enzymes and Receptors in the Neurovascular Unit: Implications in Cerebrovascular Disease. Front. Neurol. 2020, 11, 983. https://doi.org/10.3389/fneur.2020.00983.
- 154.Gonzalez-Fernandez, E.; Liu, Y.; Auchus, A.P.; Fan, F.; Roman, R.J. Vascular contributions to cognitive impairment and dementia: The emerging role of 20-HETE. Clin. Sci. 2021, 135, 1929–1944. https://doi.org/10.1042/cs20201033.
- 155.Atone, J.; Wagner, K.; Hashimoto, K.; Hammock, B.D. Cytochrome P450 derived epoxidized fatty acids as a therapeutic tool against neuroinflammatory diseases. Prostaglandins Other Lipid Mediat. 2020, 147, 106385. https://doi.org/10.1016/j.prostaglandins.2019.106385.
- 156.Davis, C.M.; Liu, X.; Alkayed, N.J. Cytochrome P450 eicosanoids in cerebrovascular function and disease. Pharmacol. Ther. 2017, 179, 31–46. https://doi.org/10.1016/j.pharmthera.2017.05.004.
- 157.Yuan, W.Q.; Huang, W.P.; Jiang, Y.C.; Xu, H.; Duan, C.S.; Chen, N.H.; Liu, Y.J.; Fu, X.M. The function of astrocytes and their role in neurological diseases. Eur. J. Neurosci. 2023, 58, 3932–3961. https://doi.org/10.1111/ejn.16160.
- 158.Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013, 8, 2003–2014.
- 159.Zhang, M.; Wang, S.; Mao, L.; Leak, R.K.; Shi, Y.; Zhang, W.; Hu, X.; Sun, B.; Cao, G.; Gao, Y. Omega-3 fatty acids protect the brain against ischemic injury by activating Nrf2 and upregulating heme oxygenase 1. J. Neurosci. 2014, 34, 1903–1915.
- 160.Fan, R.; Peng, X.; Xie, L.; Dong, K.; Ma, D.; Xu, W.; Shi, X.; Zhang, S.; Chen, J.; Yu, X.; et al. Importance of Bmal1 in Alzheimer’s disease and associated aging-related diseases: Mechanisms and interventions. Aging Cell 2022, 21, e13704. https://doi.org/10.1111/acel.13704.
- 161.Nakato, M.; Matsuo, M.; Kono, N.; Arita, M.; Arai, H.; Ogawa, J.; Kioka, N.; Ueda, K. Neurite outgrowth stimulation by n-3 and n-6 PUFAs of phospholipids in apoE-containing lipoproteins secreted from glial cells. J. Lipid Res. 2015, 56, 1880–1890.
- 162.Alessandri, J.-M.; Guesnet, P.; Vancassel, S.; Astorg, P.; Denis, I.; Langelier, B.; Aïd, S.; Poumès-Ballihaut, C.; Champeil-Potokar, G.; Lavialle, M. Polyunsaturated fatty acids in the central nervous system: Evolution of concepts and nutritional implications throughout life. Reprod. Nutr. Dev. 2004, 44, 509–538.
- 163.Lavialle, M.; Champeil-Potokar, G.; Alessandri, J.M.; Balasse, L.; Guesnet, P.; Papillon, C.; Pévet, P.; Vancassel, S.; Vivien-Roels, B.; Denis, I. An (n-3) Polyunsaturated Fatty Acid–Deficient Diet Disturbs Daily Locomotor Activity, Melatonin Rhythm, and Striatal Dopamine in Syrian Hamsters13. J. Nutr. 2008, 138, 1719–1724.
- 164.Ruggiero, C.; Lattanzio, F.; Lauretani, F.; Gasperini, B.; Andres-Lacueva, C.; Cherubini, A. Omega-3 polyunsaturated fatty acids and immune-mediated diseases: Inflammatory bowel disease and rheumatoid arthritis. Curr. Pharm. Des. 2009, 15, 4135–4148. https://doi.org/10.2174/138161209789909746.
- 165.Calder, P. PUFA, inflammatory processes and rheumatoid arthritis. Proc. Nutr. Soc. 2008, 67, 409–418.
- 166.Araújo, A.C.; Wheelock, C.E.; Haeggström, J.Z. The Eicosanoids, Redox-Regulated Lipid Mediators in Immunometabolic Disorders. Antioxid. Redox Signal 2018, 29, 275–296. https://doi.org/10.1089/ars.2017.7332.
- 167.Miyata, J.; Fukunaga, K.; Kawashima, Y.; Ohara, O.; Kawana, A.; Asano, K.; Arita, M. Dysregulated metabolism of polyunsaturated fatty acids in eosinophilic allergic diseases. Prostaglandins Other Lipid Mediat. 2020, 150, 106477. https://doi.org/10.1016/j.prostaglandins.2020.106477.
- 168.Kalinski, P. Regulation of immune responses by prostaglandin E2. J. Immunol. 2012, 188, 21–28. https://doi.org/10.4049/jimmunol.1101029.
- 169.Tsuge, K.; Inazumi, T.; Shimamoto, A.; Sugimoto, Y. Molecular mechanisms underlying prostaglandin E2-exacerbated inflammation and immune diseases. Int. Immunol. 2019, 31, 597–606. https://doi.org/10.1093/intimm/dxz021.
- 170.Henderson, W.R., Jr. The role of leukotrienes in inflammation. Ann. Intern. Med. 1994, 121, 684–697. https://doi.org/10.7326/0003-4819-121-9-199411010-00010.
- 171.Korotkova, M.; Jakobsson, P.J. Persisting eicosanoid pathways in rheumatic diseases. Nat. Rev. Rheumatol. 2014, 10, 229–241. https://doi.org/10.1038/nrrheum.2014.1.
- 172.Li, K.; Zhao, J.; Wang, M.; Niu, L.; Wang, Y.; Li, Y.; Zheng, Y. The roles of various prostaglandins in fibrosis: A review. Biomolecules 2021, 11, 789.
- 173.Serhan, C.N.; Petasis, N.A. Resolvins and protectins in inflammation resolution. Chem. Rev. 2011, 111, 5922–5943. https://doi.org/10.1021/cr100396c.
- 174.Lee, H.N.; Surh, Y.J. Therapeutic potential of resolvins in the prevention and treatment of inflammatory disorders. Biochem. Pharmacol. 2012, 84, 1340–1350. https://doi.org/10.1016/j.bcp.2012.08.004.
- 175.Morisseau, C.; Hammock, B.D. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 37–58. https://doi.org/10.1146/annurev-pharmtox-011112-140244.
- 176.Shoieb, S.M.; El-Ghiaty, M.A.; Alqahtani, M.A.; El-Kadi, A.O. Cytochrome P450-derived eicosanoids and inflammation in liver diseases. Prostaglandins Other Lipid Mediat. 2020, 147, 106400.
- 177.Hoxha, M.; Zappacosta, B. CYP-derived eicosanoids: Implications for rheumatoid arthritis. Prostaglandins Other Lipid Mediat. 2020, 146, 106405. https://doi.org/10.1016/j.prostaglandins.2019.106405.
- 178.Gilroy, D.W.; Bishop-Bailey, D. Lipid mediators in immune regulation and resolution. Br. J. Pharmacol. 2019, 176, 1009–1023. https://doi.org/10.1111/bph.14587.
- 179.Dai, M.; Wu, L.; He, Z.; Zhang, S.; Chen, C.; Xu, X.; Wang, P.; Gruzdev, A.; Zeldin, D.C.; Wang, D.W. Epoxyeicosatrienoic acids regulate macrophage polarization and prevent LPS-induced cardiac dysfunction. J. Cell Physiol. 2015, 230, 2108–2119. https://doi.org/10.1002/jcp.24939.
- 180.Man, K.; Loudon, A.; Chawla, A. Immunity around the clock. Science 2016, 354, 999–1003. https://doi.org/10.1126/science.aah4966.
- 181.Straub, R.H.; Cutolo, M. Circadian rhythms in rheumatoid arthritis: Implications for pathophysiology and therapeutic management. Arthritis Rheum. 2007, 56, 399–408. https://doi.org/10.1002/art.22368.
- 182.Cutolo, M.; Seriolo, B.; Craviotto, C.; Pizzorni, C.; Sulli, A. Circadian rhythms in RA. Ann. Rheum. Dis. 2003, 62, 593–596.
- 183.Hand, L.E.; Hopwood, T.W.; Dickson, S.H.; Walker, A.L.; Loudon, A.S.; Ray, D.W.; Bechtold, D.A.; Gibbs, J.E. The circadian clock regulates inflammatory arthritis. Faseb J. 2016, 30, 3759–3770. https://doi.org/10.1096/fj.201600353R.
- 184.Jahanban-Esfahlan, R.; Mehrzadi, S.; Reiter, R.J.; Seidi, K.; Majidinia, M.; Baghi, H.B.; Khatami, N.; Yousefi, B.; Sadeghpour, A. Melatonin in regulation of inflammatory pathways in rheumatoid arthritis and osteoarthritis: Involvement of circadian clock genes. Br. J. Pharmacol. 2018, 175, 3230–3238. https://doi.org/10.1111/bph.13898.
- 185.Norling, L.V.; Perretti, M. The role of omega-3 derived resolvins in arthritis. Curr. Opin. Pharmacol. 2013, 13, 476–481. https://doi.org/10.1016/j.coph.2013.02.003.
- 186.Cox, S.L.; O’Siorain, J.R.; Fagan, L.E.; Curtis, A.M.; Carroll, R.G. Intertwining roles of circadian and metabolic regulation of the innate immune response. Semin. Immunopathol. 2022, 44, 225–237. https://doi.org/10.1007/s00281-021-00905-5.
- 187.Kouri, V.P.; Olkkonen, J.; Kaivosoja, E.; Ainola, M.; Juhila, J.; Hovatta, I.; Konttinen, Y.T.; Mandelin, J. Circadian timekeeping is disturbed in rheumatoid arthritis at molecular level. PLoS ONE 2013, 8, e54049. https://doi.org/10.1371/journal.pone.0054049.
- 188.Gibbs, J.E.; Ray, D.W. The role of the circadian clock in rheumatoid arthritis. Arthritis Res. Ther. 2013, 15, 205. https://doi.org/10.1186/ar4146.
- 189.Dar, M.I.; Hussain, Y.; Pan, X. Roles of Circadian Clocks in Macrophage Metabolism and Cardiovascular Disease: Implications in Inflammation, and Metabolism of Lipids, Glucose, and Amino Acids. Med. Pharmacol. 2024, not peer.
- 190.Curtis, A.M.; Fagundes, C.T.; Yang, G.; Palsson-McDermott, E.M.; Wochal, P.; McGettrick, A.F.; Foley, N.H.; Early, J.O.; Chen, L.; Zhang, H.; et al. Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proc. Natl. Acad. Sci. USA 2015, 112, 7231–7236. https://doi.org/10.1073/pnas.1501327112.
- 191.Laria, A.; Lurati, A.; Marrazza, M.; Mazzocchi, D.; Re, K.A.; Scarpellini, M. The macrophages in rheumatic diseases. J. Inflamm. Res. 2016, 9, 1–11. https://doi.org/10.2147/jir.S82320.
- 192.Schouten, M.; Bielefeld, P.; Garcia-Corzo, L.; Passchier, E.M.J.; Gradari, S.; Jungenitz, T.; Pons-Espinal, M.; Gebara, E.; Martín-Suárez, S.; Lucassen, P.J.; et al. Circadian glucocorticoid oscillations preserve a population of adult hippocampal neural stem cells in the aging brain. Mol. Psychiatry 2020, 25, 1382–1405. https://doi.org/10.1038/s41380-019-0440-2.
- 193.Hua, C.; Buttgereit, F.; Combe, B. Glucocorticoids in rheumatoid arthritis: Current status and future studies. RMD Open 2020, 6, e000536. https://doi.org/10.1136/rmdopen-2017-000536.
- 194.Franzago, M.; Alessandrelli, E.; Notarangelo, S.; Stuppia, L.; Vitacolonna, E. Chrono-Nutrition: Circadian Rhythm and Personalized Nutrition. Int. J. Mol. Sci. 2023, 24, 2571. https://doi.org/10.3390/ijms24032571.
- 195.Roenneberg, T.; Allebrandt, K.V.; Merrow, M.; Vetter, C. Social jetlag and obesity. Curr. Biol. 2012, 22, 939–943. https://doi.org/10.1016/j.cub.2012.03.038.
- 196.Katsi, V.; Papakonstantinou, I.P.; Soulaidopoulos, S.; Katsiki, N.; Tsioufis, K. Chrononutrition in Cardiometabolic Health. J. Clin. Med. 2022, 11, 296. https://doi.org/10.3390/jcm11020296.
- 197.Calder, P.C. Omega-3 fatty acids and inflammatory processes. Nutrients 2010, 2, 355–374. https://doi.org/10.3390/nu2030355.
- 198.Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. N. Engl. J. Med. 1987, 317, 1736–1737.
- 199.Bonten, T.N.; Snoep, J.D.; Assendelft, W.J.; Zwaginga, J.J.; Eikenboom, J.; Huisman, M.V.; Rosendaal, F.R.; van der Bom, J.G. Time-dependent effects of aspirin on blood pressure and morning platelet reactivity: A randomized cross-over trial. Hypertension 2015, 65, 743–750. https://doi.org/10.1161/hypertensionaha.114.04980.
- 200.Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. https://doi.org/10.1056/NEJMoa1812792.
- 201.Delpino, F.M.; Figueiredo, L.M.; da Silva, B.G.C.; da Silva, T.G.; Mintem, G.C.; Bielemann, R.M.; Gigante, D.P. Omega-3 supplementation and diabetes: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 4435–4448. https://doi.org/10.1080/10408398.2021.1875977.
- 202.Brown, T.J.; Brainard, J.; Song, F.; Wang, X.; Abdelhamid, A.; Hooper, L. Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: Systematic review and meta-analysis of randomised controlled trials. BMJ 2019, 366, l4697.
- 203.Dufoo-Hurtado, E.; Wall-Medrano, A.; Campos-Vega, R. Molecular mechanisms of chronobiotics as functional foods. Mol. Mech. Funct. Food 2022, 57–86. https://doi.org/10.1002/9781119804055.ch3.
- 204.Neves, A.R.; Albuquerque, T.; Quintela, T.; Costa, D. Circadian rhythm and disease: Relationship, new insights, and future perspectives. J. Cell Physiol. 2022, 237, 3239–3256. https://doi.org/10.1002/jcp.30815.
- 205.Yurko-Mauro, K.; Alexander, D.D.; Van Elswyk, M.E. Docosahexaenoic acid and adult memory: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0120391. https://doi.org/10.1371/journal.pone.0120391.
- 206.Chang, Y.Y.; Ting, B.; Chen, D.T.; Hsu, W.T.; Lin, S.C.; Kuo, C.Y.; Wang, M.F. Omega-3 Fatty Acids for Depression in the Elderly and Patients with Dementia: A Systematic Review and Meta-Analysis. Healthcare 2024, 12, 536. https://doi.org/10.3390/healthcare12050536.
- 207.Zhang, Z.; Xin, H.; Li, M.D. Circadian rhythm of lipid metabolism in health and disease. Small Methods 2020, 4, 1900601.
- 208.Gooley, J.J.; Chua, E.C. Diurnal regulation of lipid metabolism and applications of circadian lipidomics. J. Genet. Genomics 2014, 41, 231–250. https://doi.org/10.1016/j.jgg.2014.04.001.
- 209.Li, X.; Joehanes, R.; Hoeschele, I.; Rich, S.S.; Rotter, J.I.; Levy, D.; Liu, Y.; Redline, S.; Sofer, T. Association between sleep disordered breathing and epigenetic age acceleration: Evidence from the Multi-Ethnic Study of Atherosclerosis. EBioMedicine 2019, 50, 387–394. https://doi.org/10.1016/j.ebiom.2019.11.020.
- 210.Blacher, E.; Tsai, C.; Litichevskiy, L.; Shipony, Z.; Iweka, C.A.; Schneider, K.M.; Chuluun, B.; Heller, H.C.; Menon, V.; Thaiss, C.A.; et al. Aging disrupts circadian gene regulation and function in macrophages. Nat. Immunol. 2022, 23, 229–236. https://doi.org/10.1038/s41590-021-01083-0.
- 211.Colombini, B.; Dinu, M.; Murgo, E.; Lotti, S.; Tarquini, R.; Sofi, F.; Mazzoccoli, G. Ageing and Low-Level Chronic Inflammation: The Role of the Biological Clock. Antioxidants 2022, 11, 2228. https://doi.org/10.3390/antiox11112228.
- 212.Cutuli, D. Functional and Structural Benefits Induced by Omega-3 Polyunsaturated Fatty Acids During Aging. Curr. Neuropharmacol. 2017, 15, 534–542. https://doi.org/10.2174/1570159x14666160614091311.
- 213.Külzow, N.; Witte, A.V.; Kerti, L.; Grittner, U.; Schuchardt, J.P.; Hahn, A.; Flöel, A. Impact of Omega-3 Fatty Acid Supplementation on Memory Functions in Healthy Older Adults. J. Alzheimers Dis. 2016, 51, 713–725. https://doi.org/10.3233/jad-150886.
- 214.Childs, C.E.; Romeu-Nadal, M.; Burdge, G.C.; Calder, P.C. Gender differences in the n-3 fatty acid content of tissues. Proc. Nutr. Soc. 2008, 67, 19–27. https://doi.org/10.1017/s0029665108005983.
- 215.Cutolo, M.; Sulli, A.; Capellino, S.; Villaggio, B.; Montagna, P.; Seriolo, B.; Straub, R.H. Sex hormones influence on the immune system: Basic and clinical aspects in autoimmunity. Lupus 2004, 13, 635–638. https://doi.org/10.1191/0961203304lu1094oa.
- 216.Nakamura, T.J.; Sellix, M.T.; Menaker, M.; Block, G.D. Estrogen directly modulates circadian rhythms of PER2 expression in the uterus. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1025–E1031. https://doi.org/10.1152/ajpendo.90392.2008.
- 217.Bailey, M.; Silver, R. Sex differences in circadian timing systems: Implications for disease. Front. Neuroendocrinol. 2014, 35, 111–139. https://doi.org/10.1016/j.yfrne.2013.11.003.
- 218.Škrlec, I.; Talapko, J.; Džijan, S.; Cesar, V.; Lazić, N.; Lepeduš, H. The Association between Circadian Clock Gene Polymorphisms and Metabolic Syndrome: A Systematic Review and Meta-Analysis. Biology 2021, 11, 20. https://doi.org/10.3390/biology11010020.
- 219.Ameur, A.; Enroth, S.; Johansson, A.; Zaboli, G.; Igl, W.; Johansson, A.C.; Rivas, M.A.; Daly, M.J.; Schmitz, G.; Hicks, A.A.; et al. Genetic adaptation of fatty-acid metabolism: A human-specific haplotype increasing the biosynthesis of long-chain omega-3 and omega-6 fatty acids. Am. J. Hum. Genet. 2012, 90, 809–820. https://doi.org/10.1016/j.ajhg.2012.03.014.
- 220.Brenna, J.T.; Salem, N., Jr.; Sinclair, A.J.; Cunnane, S.C. alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot. Essent. Fatty Acids 2009, 80, 85–91. https://doi.org/10.1016/j.plefa.2009.01.004.
- 221.Serhiyenko, V.; Segin, V.; Serhiyenko, A. Effects of omega-3 polyunsaturated fatty acids on the circadian rhythm of heart rate variability parameters in patients with type 2 diabetes mellitus and cardiovascular autonomic neuropathy. Российский Кардиологический журнал 2018, 5, 56–60.
- 222.Mazaherioun, M.; Djalali, M.; Koohdani, F.; Javanbakht, M.H.; Zarei, M.; Beigy, M.; Ansari, S.; Rezvan, N.; Saedisomeolia, A. Beneficial Effects of n-3 Fatty Acids on Cardiometabolic and Inflammatory Markers in Type 2 Diabetes Mellitus: A Clinical Trial. Med. Princ. Pract. 2017, 26, 535–541. https://doi.org/10.1159/000484089.
- 223.Marcheva, B.; Ramsey, K.M.; Buhr, E.D.; Kobayashi, Y.; Su, H.; Ko, C.H.; Ivanova, G.; Omura, C.; Mo, S.; Vitaterna, M.H.; et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 2010, 466, 627–631. https://doi.org/10.1038/nature09253.
- 224.Arnardottir, E.S.; Mackiewicz, M.; Gislason, T.; Teff, K.L.; Pack, A.I. Molecular signatures of obstructive sleep apnea in adults: A review and perspective. Sleep 2009, 32, 447–470. https://doi.org/10.1093/sleep/32.4.447.
- 225.Jump, D.B.; Depner, C.M.; Tripathy, S. Omega-3 fatty acid supplementation and cardiovascular disease: Thematic review series: New lipid and lipoprotein targets for the treatment of cardiometabolic diseases. J. Lipid Res. 2012, 53, 2525–2545
- 226.Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. https://doi.org/10.1016/j.bbalip.2014.08.010.
- 227.Dashti, H.S.; Follis, J.L.; Smith, C.E.; Tanaka, T.; Garaulet, M.; Gottlieb, D.J.; Hruby, A.; Jacques, P.F.; Kiefte-de Jong, J.C.; Lamon-Fava, S.; et al. Gene-Environment Interactions of Circadian-Related Genes for Cardiometabolic Traits. Diabetes Care 2015, 38, 1456–1466. https://doi.org/10.2337/dc14-2709.
- 228.Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.; Summerbell, C.D.; Worthington, H.V.; Song, F.; et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2020, 3, Cd003177. https://doi.org/10.1002/14651858.CD003177.pub5.
- 229.Maury, E. Off the clock: From circadian disruption to metabolic disease. Int. J. Mol. Sci. 2019, 20, 1597.
- 230.Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. https://doi.org/10.3390/nu8030128.
- 231.Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. https://doi.org/10.1042/bst20160474.
- 232.Lukiw, W.J.; Bazan, N.G. Docosahexaenoic acid and the aging brain. J. Nutr. 2008, 138, 2510–2514. https://doi.org/10.3945/jn.108.096016.
How to Cite
Dai, P.; Liu, J.-Y. Physiological and Pathological Insights into the Circadian Rhythm of Polyunsaturated Fatty Acids Metabolism. Health and Metabolism 2025, 2 (3), 2. https://doi.org/10.53941/hm.2025.100017.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


