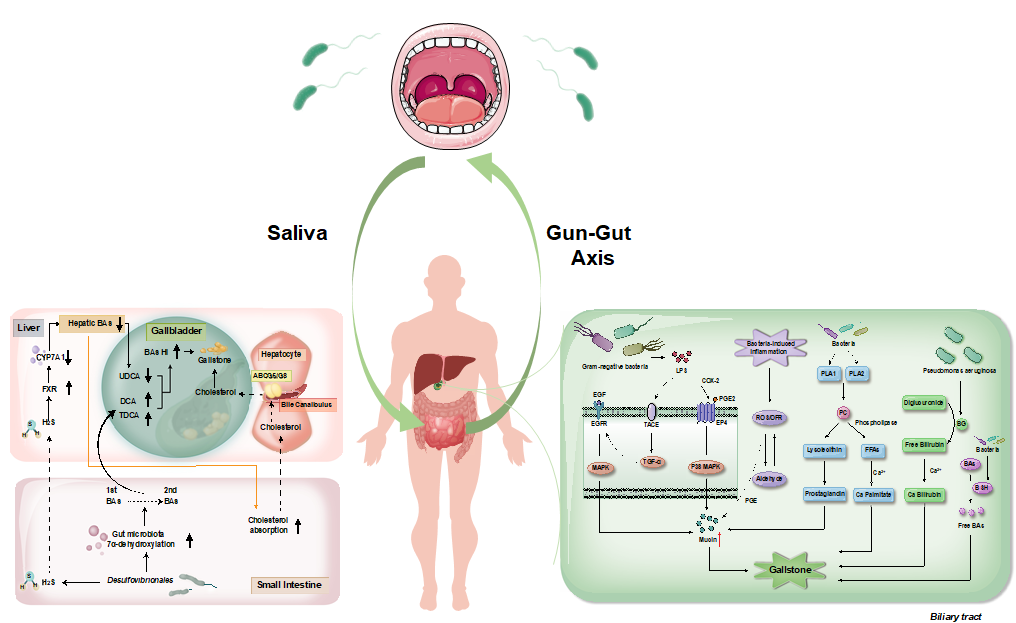
Cholelithiasis has been emerging as a pressing public health concern, entailing substantial economic burdens and oncological risks. Currently, there is a lack of effective primary and secondary prevention measures for gallstones. The etiology of gallstone formation is multifactorial, involving genetic predispositions and environmental factors. The primary pathophysiological abnormalities in gallstone formation include aberrant metabolism and secretion of cholesterol and bile acids. Cholesterol oversaturation in gallbladder bile serves as a critical precursor to gallstone pathogenesis, although the underlying mechanisms remain incompletely elucidated. Recent studies underscore the influence of gut microbiota on bile acid metabolism and gallstone formation. A notable correlation between salivary and gut microbial compositions suggests a potential ‘Gum-Gut Axis’, where translocate salivary microbes may contribute to lithogenic effects. Moreover, similarities between the microbial profiles of the biliary and duodenal regions could facilitate gallstone formation through a variety of biochemical pathways, involving β-G phospholipases, bacterial hydrolases, mucins, prostaglandins, oxygen free radicals, hydroxy sterols, LPS and metabolic shifts. This review aims to summarize the findings of correlation between gallstone pathogenesis and microbes of the digestive tract, potentially providing novel preventative or therapeutic approaches for Cholelithiasis.
- Open Access
- Review
Gastrointestinal Microbial Involvement in Gallstone Formation: A Systematic Review
- Sirui Wan 1, †,
- Kun Xia 1, †,
- Yunpeng Liu 1, 2, 3,
- Hongzhi Xu 1, 2, 3, *
Author Information
Received: 22 Feb 2025 | Revised: 20 Mar 2025 | Accepted: 25 Apr 2025 | Published: 08 Jul 2025
Abstract
Graphical Abstract
Keywords
cholelithiasis | biliary microbes | intestinal microbes | oral microbes
References
- 1.Everhart, J.E.; Ruhl, C.E. Burden of Digestive Diseases in the United States Part I: Overall and Upper Gastrointestinal Diseases. Gastroenterology 2009, 136, 376–386. https://doi.org/10.1053/j.gastro.2008.12.015.
- 2.Shaffer, E.A. Gallstone Disease: Epidemiology of Gallbladder Stone Disease. Best Pract. Res. Clin. Gastroenterol. 2006, 20, 981–996. https://doi.org/10.1016/j.bpg.2006.05.004.
- 3.Su, Z.; Gong, Y.; Liang, Z. Prevalence of Gallstone in Mainland China: A Meta-Analysis of Cross-Sectional Studies. Clin. Res. Hepatol. Gastroenterol. 2020, 44, e69–e71. https://doi.org/10.1016/j.clinre.2020.04.015.
- 4.Katsika, D.; Grjibovski, A.; Einarsson, C.; Lammert, F.; Lichtenstein, P.; Marschall, H. Genetic and Environmental Influences on Symptomatic Gallstone Disease: A Swedish Study of 43,141 Twin Pairs. Hepatology 2005, 41, 1138–1143. https://doi.org/10.1002/hep.20654.
- 5.Deng, J.; Ren, M.; Dai, X.; Qu, D.; Yang, M.; Zhang, T.; Jiang, B. Lysimachia Christinae Hance Regresses Preestablished Cholesterol Gallstone in Mice. J. Ethnopharmacol. 2015, 166, 102–108. https://doi.org/10.1016/j.jep.2015.03.031.
- 6.Larsson, S.C.; Håkansson, N.; Wolk, A. Healthy Dietary Patterns and Incidence of Biliary Tract and Gallbladder Cancer in a Prospective Study of Women and Men. Eur. J. Cancer 2017, 70, 42–47. https://doi.org/10.1016/j.ejca.2016.10.012.
- 7.Molinero, N.; Ruiz, L.; Milani, C.; Gutiérrez-Díaz, I.; Sánchez, B.; Mangifesta, M.; Segura, J.; Cambero, I.; Campelo, A.B.; García-Bernardo, C.M.; et al. The Human Gallbladder Microbiome Is Related to the Physiological State and the Biliary Metabolic Profile. Microbiome 2019, 7, 100. https://doi.org/10.1186/s40168-019-0712-8.
- 8.Hu, H.; Shao, W.; Liu, Q.; Liu, N.; Wang, Q.; Xu, J.; Zhang, X.; Weng, Z.; Lu, Q.; Jiao, L.; et al. Gut Microbiota Promotes Cholesterol Gallstone Formation by Modulating Bile Acid Composition and Biliary Cholesterol Secretion. Nat. Commun. 2022, 13, 252. https://doi.org/10.1038/s41467-021-27758-8.
- 9.Wang, H.H.; Portincasa, P.; Afdhal, N.H.; Wang, D.Q.H. Lith Genes and Genetic Analysis of Cholesterol Gallstone Formation. Gastroenterol. Clin. N. Am. 2010, 39, 185–207. https://doi.org/10.1016/j.gtc.2010.02.007.
- 10.Wu, T.; Zhang, Z.; Liu, B.; Hou, D.; Liang, Y.; Zhang, J.; Shi, P. Gut Microbiota Dysbiosis and Bacterial Community Assembly Associated with Cholesterol Gallstones in Large-Scale Study. BMC Genom. 2013, 14, 669. https://doi.org/10.1186/1471-2164-14-669.
- 11.Sung, J.Y.; Costerton, J.W.; Shaffer, E.A. Defense System in the Biliary Tract against Bacterial Infection. Dig. Dis Sci 1992, 37, 689–696. https://doi.org/10.1007/BF01296423.
- 12.Sorrentino, G.; Perino, A.; Yildiz, E.; El Alam, G.; Bou Sleiman, M.; Gioiello, A.; Pellicciari, R.; Schoonjans, K. Bile Acids Signal via TGR5 to Activate Intestinal Stem Cells and Epithelial Regeneration. Gastroenterology 2020, 159, 956–968. https://doi.org/10.1053/j.gastro.2020.05.067.
- 13.Harada, K.; Ohba, K.; Ozaki, S.; Isse, K.; Hirayama, T.; Wada, A.; Nakanuma, Y. Peptide Antibiotic Human Beta-Defensin-1 and -2 Contribute to Antimicrobial Defense of the Intrahepatic Biliary Tree. Hepatology 2004, 40, 925–932. https://doi.org/10.1002/hep.20379.
- 14.Keshavarz, M.; Faraj Tabrizi, S.; Ruppert, A.-L.; Pfeil, U.; Schreiber, Y.; Klein, J.; Brandenburger, I.; Lochnit, G.; Bhushan, S.; Perniss, A.; et al. Cysteinyl Leukotrienes and Acetylcholine Are Biliary Tuft Cell Cotransmitters. Sci. Immunol. 2022, 7, eabf6734. https://doi.org/10.1126/sciimmunol.abf6734.
- 15.Lyu, Z.; Yu, T.; Zhang, L.; Xu, X.; Zhang, Y.; Li, J.; Li, Z.; Zhang, W.; Hou, S. Analysis of the Relationship between Bile Duct and Duodenal Microbiota Reveals That Potential Dysbacteriosis Is the Main Cause of Primary Common Bile Duct Stones. Synth. Syst. Biotechnol. 2021, 6, 414–428. https://doi.org/10.1016/j.synbio.2021.11.002.
- 16.Lee, J.; Jeong, H.J.; Kim, H.; Park, J.-S. The Role of the Bile Microbiome in Common Bile Duct Stone Development. Biomedicines 2023, 11, 2124. https://doi.org/10.3390/biomedicines11082124.
- 17.Dai, C.; Xu, C.; Zheng, L.; Wang, M.; Fan, Z.; Ye, J.; Su, D. Characteristics and Metabolic Potential of Biliary Microbiota in Patients with Giant Common Bile Duct Stones. Front. Cell. Infect. Microbiol. 2023, 13, 1259761. https://doi.org/10.3389/fcimb.2023.1259761.
- 18.Song, S.-T.; Cai, L.-Y.; Zeng, X.; Xie, W.-F. Gut Microbial Profile in Asymptomatic Gallstones. Front. Microbiol. 2022, 13, 882265. https://doi.org/10.3389/fmicb.2022.882265.
- 19.Wang, Q.; Hao, C.; Yao, W.; Zhu, D.; Lu, H.; Li, L.; Ma, B.; Sun, B.; Xue, D.; Zhang, W. Intestinal Flora Imbalance Affects Bile Acid Metabolism and Is Associated with Gallstone Formation. BMC Gastroenterol. 2020, 20, 59. https://doi.org/10.1186/s12876-020-01195-1.
- 20.Jiménez, E.; Sánchez, B.; Farina, A.; Margolles, A.; Rodríguez, J.M. Characterization of the Bile and Gall Bladder Microbiota of Healthy Pigs. Microbiologyopen 2014, 3, 937–949. https://doi.org/10.1002/mbo3.218.
- 21.Shahi, S.K.; Zarei, K.; Guseva, N.V.; Mangalam, A.K. Microbiota Analysis Using Two-Step PCR and Next-Generation 16S rRNA Gene Sequencing. J. Vis. Exp. 2019, 15, 10-3791. https://doi.org/10.3791/59980.
- 22.Stewart, L.; Grifiss, J.M.; Jarvis, G.A.; Way, L.W. Biliary Bacterial Factors Determine the Path of Gallstone Formation. Am. J. Surg. 2006, 192, 598–603. https://doi.org/10.1016/j.amjsurg.2006.08.001.
- 23.Stewart, L.; Griffiss, J.M.; Jarvis, G.A.; Way, L.W. Gallstones Containing Bacteria Are Biofilms: Bacterial Slime Production and Ability to Form Pigment Solids Determines Infection Severity and Bacteremia. J. Gastrointest. Surg. 2007, 11, 977–983. https://doi.org/10.1007/s11605-007-0168-1.
- 24.Hazrah, P.; Oahn, K.T.H.; Tewari, M.; Pandey, A.K.; Kumar, K.; Mohapatra, T.M.; Shukla, H.S. The Frequency of Live Bacteria in Gallstones. HPB 2004, 6, 28–32. https://doi.org/10.1080/13651820310025192.
- 25.Kim, B.; Park, J.S.; Bae, J.; Hwang, N. Bile Microbiota in Patients with Pigment Common Bile Duct Stones. J. Korean Med. Sci. 2021, 36, e94. https://doi.org/10.3346/jkms.2021.36.e94.
- 26.Chen, B.; Fu, S.W.; Lu, L.; Zhao, H. A Preliminary Study of Biliary Microbiota in Patients with Bile Duct Stones or Distal Cholangiocarcinoma. Biomed. Res. Int. 2019, 2019, 1092563. https://doi.org/10.1155/2019/1092563.
- 27.Cai, X.; Peng, Y.; Gong, Y.; Huang, X.; Liu, L.; Chen, Y.; Du, J.; Dai, Z.; Qian, Y.; Xu, L. Variations of Bile Bacterial Community alongside Gallstone Disease Progression and Key Taxa Involved in Poor Outcomes after Endoscopic Surgery. Eur. J. Med. Res. 2023, 28, 313. https://doi.org/10.1186/s40001-023-01308-y.
- 28.Choi, S.J.; Kim, Y.; Jeon, J.; Gwak, H.J.; Kim, M.; Kang, K.; Kim, Y.; Jeong, J.; Jung, Y.K.; Lee, K.G.; et al. Association of Microbial Dysbiosis with Gallbladder Diseases Identified by Bile Microbiome Profiling. J. Korean Med. Sci. 2021, 36, e189. https://doi.org/10.3346/jkms.2021.36.e189.
- 29.Feng, R.; Zhang, T.; Kayani, M.U.R.; Wang, Z.; Shen, Y.; Su, K.L.; Bielike, K.; Chen, L. Patients with Primary and Secondary Bile Duct Stones Harbor Distinct Biliary Microbial Composition and Metabolic Potential. Front. Cell. Infect. Microbiol. 2022, 12, 881489. https://doi.org/10.3389/fcimb.2022.881489.
- 30.Liu, Q.; Zheng, L.; Wang, Y.; Huang, Z.; Zhu, J.; Fang, M.; Xie, L.; Ding, C.; Gu, Y.; Xu, D.; et al. Primary Choledocholithiasis Occurrence and Recurrence Is Synergetcally Modulated by the Bile Microbiome and Metabolome Alternations. Life Sci. 2023, 331, 122073. https://doi.org/10.1016/j.lfs.2023.122073.
- 31.Ye, F.; Shen, H.; Li, Z.; Meng, F.; Li, L.; Yang, J.; Chen, Y.; Bo, X.; Zhang, X.; Ni, M. Influence of the Biliary System on Biliary Bacteria Revealed by Bacterial Communities of the Human Biliary and Upper Digestive Tracts. PLoS ONE 2016, 11, e0150519. https://doi.org/10.1371/journal.pone.0150519.
- 32.Liu, L.; Zhao, Z.; Hou, X.; Wu, J. Effect of Sphincter of Oddi Dysfunction on the Abundance of Biliary Microbiota (Biliary Microecology) in Patients with Common Bile Duct Stones. Front. Cell. Infect. Microbiol. 2022, 12, 1001441. https://doi.org/10.3389/fcimb.2022.1001441.
- 33.Lomovskaya, O.; Lewis, K. Emr, an Escherichia Coli Locus for Multidrug Resistance. Proc. Natl. Acad. Sci. USA 1992, 89, 8938–8942. https://doi.org/10.1073/pnas.89.19.8938.
- 34.Lomovskaya, O.; Lewis, K.; Matin, A. EmrR Is a Negative Regulator of the Escherichia Coli Multidrug Resistance Pump EmrAB. J. Bacteriol. 1995, 177, 2328–2334. https://doi.org/10.1128/jb.177.9.2328-2334.1995.
- 35.Kullak-Ublick, G.A.; Stieger, B.; Meier, P.J. Enterohepatic Bile Salt Transporters in Normal Physiology and Liver Disease. Gastroenterology 2004, 126, 322–342. https://doi.org/10.1053/j.gastro.2003.06.005.
- 36.Liu, B.; Zhuang, S.; Tian, R.; Liu, Y.; Wang, Y.; Lei, X.; Wang, C. Chemoproteomic Profiling Reveals the Mechanism of Bile Acid Tolerance in Bacteria. ACS Chem. Biol. 2022, 17, 2461–2470. https://doi.org/10.1021/acschembio.2c00286.
- 37.González, J.F.; Alberts, H.; Lee, J.; Doolittle, L.; Gunn, J.S. Biofilm Formation Protects Salmonella from the Antibiotic Ciprofloxacin In Vitro and In Vivo in the Mouse Model of Chronic Carriage. Sci. Rep. 2018, 8, 222. https://doi.org/10.1038/s41598-017-18516-2.
- 38.Silva, C.P.; Pereira-Lima, J.C.; Oliveira, A.G.; Guerra, J.B.; Marques, D.L.; Sarmanho, L.; Cabral, M.M.D.A.; Queiroz, D.M.M. Association of the Presence of Helicobacter in Gallbladder Tissue with Cholelithiasis and Cholecystitis. J. Clin. Microbiol. 2003, 41, 5615–5618. https://doi.org/10.1128/JCM.41.12.5615-5618.2003.
- 39.Liu, Z.; Kemp, T.J.; Gao, Y.-T.; Corbel, A.; McGee, E.E.; Wang, B.; Shen, M.-C.; Rashid, A.; Hsing, A.W.; Hildesheim, A.; et al. Association of Circulating Inflammation Proteins and Gallstone Disease. J. Gastroenterol. Hepatol. 2018, 33, 1920–1924. https://doi.org/10.1111/jgh.14265.
- 40.Woof, J.M.; Mestecky, J. Mucosal Immunoglobulins. Immunol. Rev. 2005, 206, 64–82. https://doi.org/10.1111/j.0105-2896.2005.00290.x.
- 41.Geetha, A. Evidence for Oxidative Stress in the Gall Bladder Mucosa of Gall Stone Patients. J. Biochem. Mol. Biol. Biophys. 2002, 6, 427–432. https://doi.org/10.1080/1025814021000036179.
- 42.Eder, M.I.; Miquel, J.F.; Jongst, D.; Paumgartner, G.; von Ritter, C. Reactive Oxygen Metabolites Promote Cholesterol Crystal Formation in Model Bile: Role of Lipid Peroxidation. Free Radic. Biol. Med. 1996, 20, 743–749. https://doi.org/10.1016/0891-5849(95)02154-x.
- 43.Hale, W.B.; Turner, B.; LaMont, J.T. Oxygen Radicals Stimulate Guinea Pig Gallbladder Glycoprotein Secretion in Vitro. Am. J. Physiol. 1987, 253, G627–G630. https://doi.org/10.1152/ajpgi.1987.253.5.G627.
- 44.Liu, P.; Xiao, L.; Chen, J. Alterations of oxygen free radicals in rabbit models and its relation to formation of pigment gallstones. Hua Xi Yi Ke Da Xue Xue Bao 1994, 25, 406–409.
- 45.Maki, T. Pathogenesis of Calcium Bilirubinate Gallstone: Role of E. Coli, Beta-Glucuronidase and Coagulation by Inorganic Ions, Polyelectrolytes and Agitation. Ann. Surg. 1966, 164, 90–100. https://doi.org/10.1097/00000658-196607000-00010.
- 46.LaMorte, W.W.; Booker, M.L.; Scott, T.E.; Williams, L.F. Increases in Gallbladder Prostaglandin Synthesis before the Formation of Cholesterol Gallstones. Surgery 1985, 98, 445–451.
- 47.Swidsinski, A.; Lee, S.P. The Role of Bacteria in Gallstone Pathogenesis. Front. Biosci. 2001, 6, E93–E103. https://doi.org/10.2741/swidsinski.
- 48.Kim, H.-J.; Lee, S.-K.; Kim, M.-H.; Seo, D.-W.; Min, Y.-I. Cyclooxygenase-2 Mediates Mucin Secretion from Epithelial Cells of Lipopolysaccharide-Treated Canine Gallbladder. Dig. Dis. Sci. 2003, 48, 726–732. https://doi.org/10.1023/a:1022832608466.
- 49.Bar Dayan, Y.; Vilkin, A.; Niv, Y. Gallbladder Mucin Plays a Role in Gallstone Formation. Eur. J. Intern. Med. 2004, 15, 411–414. https://doi.org/10.1016/j.ejim.2004.07.010.
- 50.Smith, B.F.; LaMont, J.T. Identification of Gallbladder Mucin-Bilirubin Complex in Human Cholesterol Gallstone Matrix. Effects of Reducing Agents on in Vitro Dissolution of Matrix and Intact Gallstones. J. Clin. Investig. 1985, 76, 439–445. https://doi.org/10.1172/JCI111991.
- 51.Yoo, K.-S.; Choi, H.S.; Jun, D.W.; Lee, H.L.; Lee, O.Y.; Yoon, B.C.; Lee, K.G.; Paik, S.S.; Kim, Y.S.; Lee, J. MUC Expression in Gallbladder Epithelial Tissues in Cholesterol-Associated Gallbladder Disease. Gut Liver 2016, 10, 851–858. https://doi.org/10.5009/gnl15600.
- 52.Di Ciaula, A.; Wang, D.Q.-H.; Portincasa, P. An Update on the Pathogenesis of Cholesterol Gallstone Disease. Curr. Opin. Gastroenterol. 2018, 34, 71–80. https://doi.org/10.1097/MOG.0000000000000423.
- 53.Lei, Y.-M.; Yan, R.; Gao, Y.-D.; Yang, H.-J.; Bi, H.-Y.; Duan, Y.-Q. Cholesterol Crystals Activate NLRP3 Inflammasomes and Promote Gallstone Formation by Increasing Mucin Secretion. Biotech. Histochem. 2022, 97, 546–553. https://doi.org/10.1080/10520295.2022.2036813.
- 54.Peng, Y.; Yang, Y.; Liu, Y.; Nie, Y.; Xu, P.; Xia, B.; Tian, F.; Sun, Q. Cholesterol Gallstones and Bile Host Diverse Bacterial Communities with Potential to Promote the Formation of Gallstones. Microb. Pathog. 2015, 83–84, 57–63. https://doi.org/10.1016/j.micpath.2015.05.002.
- 55.Caldwcll, F.T.; Levitsky, K. The Gallbladder and Gallstone Formation: Ann. Surg. 1967, 166, 753–758. https://doi.org/10.1097/00000658-196711000-00003.
- 56.Ding, L.; Wang, S.; Jiang, W.; Miao, Y.; Liu, W.; Yang, F.; Zhang, J.; Chi, W.; Liu, T.; Liu, Y.; et al. Identification of Intestinal Microbial Community in Gallstone Patients with Metagenomic Next-Generation Sequencing. Diagnostics 2023, 13, 2712. https://doi.org/10.3390/diagnostics13162712.
- 57.Zhang, T.; Zhang, S.; Jin, C.; Lin, Z.; Deng, T.; Xie, X.; Deng, L.; Li, X.; Ma, J.; Ding, X.; et al. A Predictive Model Based on the Gut Microbiota Improves the Diagnostic Effect in Patients with Cholangiocarcinoma. Front. Cell. Infect. Microbiol. 2021, 11, 751795. https://doi.org/10.3389/fcimb.2021.751795.
- 58.Georgescu, D.; Ionita, I.; Lascu, A.; Hut, E.-F.; Dragan, S.; Ancusa, O.-E.; Ionita, M.; Calamar-Popovici, D.; Georgescu, L.-A.; Lighezan, D.-F. Gallstone Disease and Bacterial Metabolic Performance of Gut Microbiota in Middle-Aged and Older Patients. IJGM 2022, 15, 5513–5531. https://doi.org/10.2147/IJGM.S350104.
- 59.dos Santos, J.S.; Júnior, W.S.; Módena, J.L.P.; Brunaldi, J.E.; Ceneviva, R. Effect of Preoperative Endoscopic Decompression on Malignant Biliary Obstruction and Postoperative Infection. Hepatogastroenterology 2005, 52, 45–47.
- 60.Ipek, S.; Alper, E.; Cekic, C.; Cerrah, S.; Arabul, M.; Aslan, F.; Unsal, B. Evaluation of the Effectiveness of Endoscopic Retrograde Cholangiopancreatography in Patients with Perihilar Cholangiocarcinoma and Its Effect on Development of Cholangitis. Gastroenterol. Res. Pract. 2014, 2014, 508286. https://doi.org/10.1155/2014/508286.
- 61.Tan, W.; Chen, R.; Song, J.; He, D.; Wu, J.; Chen, X.; Yang, X.; Ye, L. Microbiota Analysis with Next-Generation 16S rDNA Gene Sequencing in Recurrent Common Bile Duct Stones. Ann. Transl. Med. 2022, 10, 576. https://doi.org/10.21037/atm-22-2247.
- 62.Frost, F.; Kacprowski, T.; Rühlemann, M.; Weiss, S.; Bang, C.; Franke, A.; Pietzner, M.; Aghdassi, A.A.; Sendler, M.; Völker, U.; et al. Carrying Asymptomatic Gallstones Is Not Associated with Changes in Intestinal Microbiota Composition and Diversity but Cholecystectomy with Significant Dysbiosis. Sci. Rep. 2021, 11, 6677. https://doi.org/10.1038/s41598-021-86247-6.
- 63.Wang, D.Q.-H.; Cohen, D.E.; Carey, M.C. Biliary Lipids and Cholesterol Gallstone Disease. J. Lipid Res. 2009, 50, S406–S411. https://doi.org/10.1194/jlr.R800075-JLR200.
- 64.Petrov, V.A.; Fernández-Peralbo, M.A.; Derks, R.; Knyazeva, E.M.; Merzlikin, N.V.; Sazonov, A.E.; Mayboroda, O.A.; Saltykova, I.V. Biliary Microbiota and Bile Acid Composition in Cholelithiasis. BioMed Res. Int. 2020, 2020, 1242364. https://doi.org/10.1155/2020/1242364.
- 65.Molinero, N.; Ruiz, L.; Sánchez, B.; Margolles, A.; Delgado, S. Intestinal Bacteria Interplay with Bile and Cholesterol Metabolism: Implications on Host Physiology. Front. Physiol. 2019, 10, 185. https://doi.org/10.3389/fphys.2019.00185.
- 66.Swann, J.R.; Want, E.J.; Geier, F.M.; Spagou, K.; Wilson, I.D.; Sidaway, J.E.; Nicholson, J.K.; Holmes, E. Systemic Gut Microbial Modulation of Bile Acid Metabolism in Host Tissue Compartments. Proc. Natl. Acad. Sci. USA 2011, 108, 4523–4530. https://doi.org/10.1073/pnas.1006734107.
- 67.Sayin, S.I.; Wahlström, A.; Felin, J.; Jäntti, S.; Marschall, H.-U.; Bamberg, K.; Angelin, B.; Hyötyläinen, T.; Orešič, M.; Bäckhed, F. Gut Microbiota Regulates Bile Acid Metabolism by Reducing the Levels of Tauro-Beta-Muricholic Acid, a Naturally Occurring FXR Antagonist. Cell Metab. 2013, 17, 225–235. https://doi.org/10.1016/j.cmet.2013.01.003.
- 68.Chen, M.L.; Takeda, K.; Sundrud, M.S. Emerging Roles of Bile Acids in Mucosal Immunity and Inflammation. Mucosal Immunol. 2019, 12, 851–861. https://doi.org/10.1038/s41385-019-0162-4.
- 69.Wang, L.; Guo, M.-J.; Gao, Q.; Yang, J.-F.; Yang, L.; Pang, X.-L.; Jiang, X.-J. The Effects of Probiotics on Total Cholesterol: A Meta-Analysis of Randomized Controlled Trials. Medicine 2018, 97, e9679. https://doi.org/10.1097/MD.0000000000009679.
- 70.Jones, M.L.; Martoni, C.J.; Parent, M.; Prakash, S. Cholesterol-Lowering Efficacy of a Microencapsulated Bile Salt Hydrolase-Active Lactobacillus Reuteri NCIMB 30242 Yoghurt Formulation in Hypercholesterolaemic Adults. Br. J. Nutr. 2012, 107, 1505–1513. https://doi.org/10.1017/S0007114511004703.
- 71.Schneider, K.M.; Candels, L.S.; Hov, J.R.; Myllys, M.; Hassan, R.; Schneider, C.V.; Wahlström, A.; Mohs, A.; Zühlke, S.; Liao, L.; et al. Gut Microbiota Depletion Exacerbates Cholestatic Liver Injury via Loss of FXR Signalling. Nat. Metab. 2021, 3, 1228–1241. https://doi.org/10.1038/s42255-021-00452-1.
- 72.Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-Fat-Induced Taurocholic Acid Promotes Pathobiont Expansion and Colitis in Il10-/- Mice. Nature 2012, 487, 104–108. https://doi.org/10.1038/nature11225.
- 73.Dan, W.-Y.; Yang, Y.-S.; Peng, L.-H.; Sun, G.; Wang, Z.-K. Gastrointestinal Microbiome and Cholelithiasis: Current Status and Perspectives. World J. Gastroenterol. 2023, 29, 1589–1601. https://doi.org/10.3748/wjg.v29.i10.1589.
- 74.Gao, L.; Xu, T.; Huang, G.; Jiang, S.; Gu, Y.; Chen, F. Oral Microbiomes: More and More Importance in Oral Cavity and Whole Body. Protein Cell 2018, 9, 488–500. https://doi.org/10.1007/s13238-018-0548-1.
- 75.Shen, H.; Ye, F.; Xie, L.; Yang, J.; Li, Z.; Xu, P.; Meng, F.; Li, L.; Chen, Y.; Bo, X.; et al. Metagenomic Sequencing of Bile from Gallstone Patients to Identify Different Microbial Community Patterns and Novel Biliary Bacteria. Sci. Rep. 2015, 5, 17450. https://doi.org/10.1038/srep17450.
- 76.Maki, K.A.; Kazmi, N.; Barb, J.J.; Ames, N. The Oral and Gut Bacterial Microbiomes: Similarities, Differences, and Connections. Biol. Res. Nurs. 2021, 23, 7–20. https://doi.org/10.1177/1099800420941606.
- 77.Byrd, K.M.; Gulati, A.S. The “Gum–Gut” Axis in Inflammatory Bowel Diseases: A Hypothesis-Driven Review of Associations and Advances. Front. Immunol. 2021, 12, 620124. https://doi.org/10.3389/fimmu.2021.620124.
- 78.Lourenςo, T.G.B.; Spencer, S.J.; Alm, E.J.; Colombo, A.P.V. Defining the Gut Microbiota in Individuals with Periodontal Diseases: An Exploratory Study. J. Oral Microbiol. 2018, 10, 1487741. https://doi.org/10.1080/20002297.2018.1487741.
- 79.Schmidt, T.S.; Hayward, M.R.; Coelho, L.P.; Li, S.S.; Costea, P.I.; Voigt, A.Y.; Wirbel, J.; Maistrenko, O.M.; Alves, R.J.; Bergsten, E.; et al. Extensive Transmission of Microbes along the Gastrointestinal Tract. eLife 2019, 8, e42693. https://doi.org/10.7554/eLife.42693.
- 80.Mall, A.S.; Habte, H.; Mthembu, Y.; Peacocke, J.; De Beer, C. Mucus and Mucins: Do They Have a Role in the Inhibition of the Human Immunodeficiency Virus? Virol. J. 2017, 14, 192. https://doi.org/10.1186/s12985-017-0855-9.
- 81.Walker, M.Y.; Pratap, S.; Southerland, J.H.; Farmer-Dixon, C.M.; Lakshmyya, K.; Gangula, P.R. Role of Oral and Gut Microbiome in Nitric Oxide-Mediated Colon Motility. Nitric Oxide 2018, 73, 81–88. https://doi.org/10.1016/j.niox.2017.06.003.
- 82.Palazzo, M.; Balsari, A.; Rossini, A.; Selleri, S.; Calcaterra, C.; Gariboldi, S.; Zanobbio, L.; Arnaboldi, F.; Shirai, Y.F.; Serrao, G.; et al. Activation of Enteroendocrine Cells via TLRs Induces Hormone, Chemokine, and Defensin Secretion. J. Immunol. 2007, 178, 4296–4303. https://doi.org/10.4049/jimmunol.178.7.4296.
- 83.Gutt, C.; Schläfer, S.; Lammert, F. The Treatment of Gallstone Disease. Dtsch. Arztebl. Int. 2020, 117, 148–158. https://doi.org/10.3238/arztebl.2020.0148.
- 84.Di Ciaula, A.; Wang, D.Q.-H.; Portincasa, P. Cholesterol Cholelithiasis: Part of a Systemic Metabolic Disease, Prone to Primary Prevention. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 157–171. https://doi.org/10.1080/17474124.2019.1549988.
- 85.Choi, J.H.; Lee, S.H.; Cho, I.R.; Paik, W.H.; Ryu, J.K.; Kim, Y.-T. Ursodeoxycholic Acid for the Prevention of Gallstone and Subsequent Cholecystectomy Following Gastric Surgery: A Systematic Review and Meta-Analysis. J. Hepato. Pancreat. Sci. 2021, 28, 409–418. https://doi.org/10.1002/jhbp.946.
- 86.Rosa, L.; Lobos-González, L.; Muñoz-Durango, N.; García, P.; Bizama, C.; Gómez, N.; González, X.; Wichmann, I.A.; Saavedra, N.; Guevara, F.; et al. Evaluation of the Chemopreventive Potentials of Ezetimibe and Aspirin in a Novel Mouse Model of Gallbladder Preneoplasia. Mol. Oncol. 2020, 14, 2834–2852. https://doi.org/10.1002/1878-0261.12766.
- 87.Ye, X.; Huang, D.; Dong, Z.; Wang, X.; Ning, M.; Xia, J.; Shen, S.; Wu, S.; Shi, Y.; Wang, J.; et al. FXR Signaling-Mediated Bile Acid Metabolism Is Critical for Alleviation of Cholesterol Gallstones by Lactobacillus Strains. Microbiol. Spectr. 2022, 10, e0051822. https://doi.org/10.1128/spectrum.00518-22.
- 88.Chen, Q.; Zhang, Y.; Li, S.; Chen, S.; Lin, X.; Li, C.; Asakawa, T. Mechanisms Underlying the Prevention and Treatment of Cholelithiasis Using Traditional Chinese Medicine. Evid. -Based Complement. Altern. Med. 2019, 2019, 2536452. https://doi.org/10.1155/2019/2536452.
- 89.Bajaj, J.S.; Hays, R.A. Manipulation of the Gut-Liver Axis Using Microbiome Restoration Therapy in Primary Sclerosing Cholangitis. Am. J. Gastroenterol. 2019, 114, 1027–1029. https://doi.org/10.14309/ajg.0000000000000191.
- 90.Bustamante, J.-M.; Dawson, T.; Loeffler, C.; Marfori, Z.; Marchesi, J.R.; Mullish, B.H.; Thompson, C.C.; Crandall, K.A.; Rahnavard, A.; Allegretti, J.R.; et al. Impact of Fecal Microbiota Transplantation on Gut Bacterial Bile Acid Metabolism in Humans. Nutrients 2022, 14, 5200. https://doi.org/10.3390/nu14245200.
- 91.Shen, S.; Huang, D.; Qian, S.; Ye, X.; Zhuang, Q.; Wan, X.; Dong, Z. Hyodeoxycholic Acid Attenuates Cholesterol Gallstone Formation via Modulation of Bile Acid Metabolism and Gut Microbiota. Eur. J. Pharmacol. 2023, 955, 175891. https://doi.org/10.1016/j.ejphar.2023.175891.
- 92.Lu, Q.; Jiang, Z.; Wang, Q.; Hu, H.; Zhao, G. The Effect of Tauroursodeoxycholic Acid (TUDCA) and Gut Microbiota on Murine Gallbladder Stone Formation. Ann. Hepatol. 2021, 23, 100289. https://doi.org/10.1016/j.aohep.2020.100289.
- 93.Shen, W.; Shao, W.; Wang, Q.; Wang, B.; Zhao, G.; Gu, A.; Jiang, Z.; Hu, H. Dietary Diosgenin Transcriptionally Down-Regulated Intestinal NPC1L1 Expression to Prevent Cholesterol Gallstone Formation in Mice. J. Biomed. Sci. 2023, 30, 44. https://doi.org/10.1186/s12929-023-00933-3.
- 94.Liu, S.; Luorong, Q.; Hu, K.; Cao, W.; Tao, W.; Liu, H.; Zhang, D. Aqueous Extract of Lysimachia Christinae Hance Prevents Cholesterol Gallstone in Mice by Affecting the Intestinal Microflora. J. Microbiol. Biotechnol. 2021, 31, 1272–1280. https://doi.org/10.4014/jmb.2106.06043.
- 95.Lammert, F.; Gurusamy, K.; Ko, C.W.; Miquel, J.-F.; Méndez-Sánchez, N.; Portincasa, P.; van Erpecum, K.J.; van Laarhoven, C.J.; Wang, D.Q.-H. Gallstones. Nat. Rev. Dis. Primers 2016, 2, 16024. https://doi.org/10.1038/nrdp.2016.24.
- 96.Lee, N.Y.; Shin, M.J.; Youn, G.S.; Yoon, S.J.; Choi, Y.R.; Kim, H.S.; Gupta, H.; Han, S.H.; Kim, B.K.; Lee, D.Y.; et al. Lactobacillus Attenuates Progression of Nonalcoholic Fatty Liver Disease by Lowering Cholesterol and Steatosis. Clin. Mol. Hepatol. 2021, 27, 110–124. https://doi.org/10.3350/cmh.2020.0125.
- 97.Khare, A.; Gaur, S. Cholesterol-Lowering Effects of Lactobacillus Species. Curr. Microbiol. 2020, 77, 638–644. https://doi.org/10.1007/s00284-020-01903-w.
- 98.Liu, Y.; Li, H.; Sun, T.; Sun, G.; Jiang, B.; Liu, M.; Wang, Q.; Li, T.; Cao, J.; Zhao, L.; et al. Gut Microbiome and Metabolome Characteristics of Patients with Cholesterol Gallstones Suggest the Preventive Potential of Prebiotics. iMeta 2025, 4, e70000. https://doi.org/10.1002/imt2.70000.
- 99.Hughes, R.L.; Alvarado, D.A.; Swanson, K.S.; Holscher, H.D. The Prebiotic Potential of Inulin-Type Fructans: A Systematic Review. Adv. Nutr. 2022, 13, 492–529. https://doi.org/10.1093/advances/nmab119.
- 100.Berlutti, F.; Pilloni, A.; Pietropaoli, M.; Polimeni, A.; Valenti, P. Lactoferrin and Oral Diseases: Current Status and Perspective in Periodontitis. Ann. Stomatol. 2011, 2, 10–18.
How to Cite
Wan, S.; Xia, K.; Liu, Y.; Xu, H. Gastrointestinal Microbial Involvement in Gallstone Formation: A Systematic Review. Health and Metabolism 2025, 2 (3), 3. https://doi.org/10.53941/hm.2025.100018.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


