SAP is a serum protein associated with an increased risk of cardiovascular events, and studies have uncovered a significant positive correlation between SAP and the pathogenesis of atherosclerosis. Our previous research found that SAP deficiency in ApoE−/− mice significantly reduced the formation of foam cells and the expression of pro-inflammatory factors, and caused significant metabolic changes. However, it is still unclear whether SAP deficiency has the same function and metabolic regulation effect on normal background mice. In this study, C57 and SAP−/− mice were used as models. Histopathological examinations were performed to evaluate the histomorphological changes of heart, liver, spleen, lung and kidney tissues in SAP−/− mice. The results showed that there were no significant histopathology changes in the main organs of SAP−/− mice, but there were significant differences in the serum metabolic profiles between C57 and SAP−/− mice. It was found that the deficiency of SAP in both C57 and ApoE−/− mice upregulated choline, but the regulatory trends on LDL/VLDL were different. Acetate and pyruvate were regulated by SAP deficiency in ApoE−/− mice, but not in C57 mice. In addition, the deletion of SAP also caused significant changes in formate, tyrosine, glycine, glucose, TMAO, taurine, citrate, succinate, and histidine in C57 context, which were not detected in the context of atherosclerosis. In summary, this study indicates that the metabolic regulation of the SAP gene is not only related to the gene itself but also related to the physiological or pathological state of the organism.
- Open Access
- Article
Comparative Study on Metabolic Regulation of SAP Deletion in Normal Mice and Atherosclerotic Mice
- Yifan Zhu 1,
- Shuhan Xie 2, †,
- Hailong Shu 3, †,
- Yiqing Lu 4,
- Jiawei Guo 4,
- Qian Li 2,
- Yongli Zhang 2,
- Yongxia Yang 3, *
Author Information
Received: 25 Dec 2024 | Revised: 13 Jan 2025 | Accepted: 27 Jun 2025 | Published: 11 Jul 2025
Abstract
Graphical Abstract
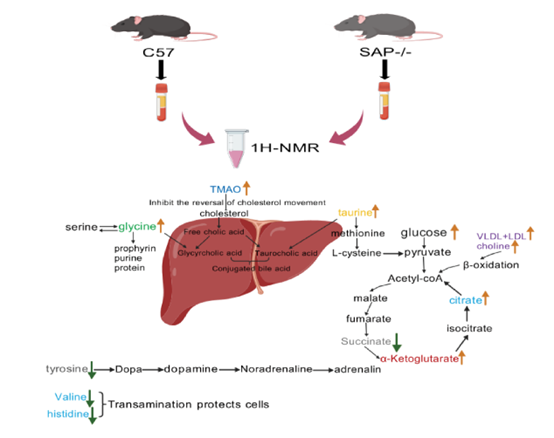
Keywords
1H-NMR | metabolomics | atherosclerotic | Serum amyloid P protein (SAP)
References
- 1.Loveless; R.W.; Floyd-O’Sullivan; Raynes; J.G.; Yuen; Feizi, T. Human serum amyloid P is a multispecific adhesive protein whose ligands include 6-phosphorylated mannose and the 3-sulphated saccharides galactose, N-acetylgalactosamine and glucuronic acid. EMBO J. 1992, 11, 813–819.
- 2.Jenny; N.S.; Arnold; A.M.; Kuller; L.H.; Tracy; R.P.; Psaty, B.M. Serum amyloid P and cardiovascular disease in older men and women: Results from the Cardiovascular Health Study. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 352–358.
- 3.D.; Luo; T.; Xiong; H.; Liu; J.; Lu; H.; Li; M.; Hou; Y.; Guo; SAP, Z.; function, and its roles in immune-related diseases. Int. J. Cardiol. 2015, 187, 20–26.
- 4.Song; Z.; Cai; L.; Guo; L.; Tsukamoto; Y.; Yutani; C.; Li, X. Accumulation and expression of serum amyloid P component in human atherosclerotic lesions. Atherosclerosis 2010, 211, 90–95.
- 5.Cox; N.; Pilling; D.; Gomer, R.H. Serum amyloid P: A systemic regulator of the innate immune response. J. Leukoc. Biol. 2014, 96, 739–743.
- 6.Hutchinson, W.L.; Hohenester, E.; Pepys, M.B. Human serum amyloid P component is a single uncomplexed pentamer in whole serum. Mol. Med. 2000, 6, 482–493.
- 7.Pilely, K.; Fumagalli, S.; Rosbjerg, A.; Genster, N.; Skjoedt, M.; Perego, C.; Ferrante, A.M.R.; Simoni, M.D.; Garred, P. C-reactive protein binds to cholesterol crystals and co-localizes with the terminal complement complex in human atherosclerotic plaques. Front. Immunol. 2017, 8, 1040.
- 8.Gisterå, A.; Hansson, G.K. The immunology of atherosclerosis. Nat. Rev. Nephrol. 2017, 13, 368–380.
- 9.Richards, D.B.; Cookson, L.M.; Berges, A.C.; Barton, S.V.; Lane, T.; Ritter, J.M.; Fontana, M.; Moon, J.C.; Pinzani, M.; Gillmore, J.D.; et al. Therapeutic clearance of amyloid by antibodies to serum amyloid P component. N. Engl. J. Med. 2015, 373, 1106–1114.
- 10.Desai, H.V.; Aronow, W.S.; Peterson, S.J.; Frishman, W.H. Cardiac amyloidosis: Approaches to diagnosis and management. Cardiol. Rev. 2010, 18, 1–11.
- 11.Pepys, M.B. Pathogenesis, diagnosis and treatment of systemic amyloidosis. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 2001, 356, 203–211.
- 12.Li, X.A.; Hatanaka, K.; Ishibashi-Ueda, H.; Yutani, C.; Yamamoto, A. Characterization of serum amyloid P component from human aortic atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 252–257.
- 13.Zheng, L.; Wu, T.; Zeng, C.; Li, X.; Li, X.; Wen, D.; Ji, T.; Lan, T.; Xing, L.; Li, J.; et al. SAP deficiency mitigated atherosclerotic lesions in ApoE−/− mice. Atherosclerosis 2016, 244, 179–187.
- 14.Li, Q.; Chen, W.; Huang, W.; Hou, R.; Huang, X.; Xu, M.; Que, L.; Wang, L.; Yang, Y. 1H-NMR-Based Metabonomics Study to Reveal the Progressive Metabolism Regulation of SAP Deficiency on ApoE−/− Mice. Metabolites 2022, 12, 1278.
- 15.Iida, M.; Harada, S.; Takebayashi, T. Application of metabolomics to epidemiological studies of atherosclerosis and cardiovascular disease. J. Atheroscler. Thromb. 2019, 26, 747–757.
- 16.Crook, A.A.; Powers, R. Quantitative NMR-based biomedical metabolomics: Current status and applications. Molecules 2020, 25, 5128.
- 17.Tzoulaki, I.; Castagné, R.; Boulangé, C.L.; Karaman, I.; Chekmeneva, E.; Evangelou, E.; Ebbels, T.M.D.; Kaluarachchi, M.R.; Chadeau-Hyam, M.; Mosen, D.; et al. Serum metabolic signatures of coronary and carotid atherosclerosis and subsequent cardiovascular disease. Eur. Heart J. 2019, 40, 2883–2896.
- 18.Li, B.; Lu, X.; Wang, J.; He, X.; Gu, Q.; Wang, L.; Yang, Y. The metabonomics study of P-selectin glycoprotein ligand-1 (PSGL-1) deficiency inhibiting the progression of atherosclerosis in LDLR−/− mice. Int. J. Biol. Sci. 2018, 14, 36–46.
- 19.Chen, W.; Li, Q.; Hou, R.; Liang, H.; Zhang, Y.; Yang, Y. An integrated metabonomics study to reveal the inhibitory effect and metabolism regulation of taurine on breast cancer. J. Pharm. Biomed. Anal. 2022, 214, 114711.
- 20.Mora-Ortiz, M.; Ramos, P.N.; Oregioni, A.; Claus, S.P. NMR metabolomics identifies over 60 biomarkers associated with Type II Diabetes impairment in db/db mice Metabolomics. Off. J. Metabolomic Soc. 2019, 15, 89.
- 21.Meng, Z.; Tian, S.; Yan, J.; Jia, M.; Yan, S.; Li, R.; Zhang, R.; Zhu, W.; Zhou, Z. Effects of perinatal exposure to BPA, BPF and BPAF on liver function in male mouse offspring involving in oxidative damage and metabolic disorder. Environ. Pollut. 2019, 247, 935–943.
- 22.Jin, M.; Pan, T.; Tocher, D.R.; Betancor, M.B.; Monroig, Ó.; Shen, Y.; Zhu, T.; Sun, P.; Jia, L.; Zhou, Q. Dietary choline supplementation attenuated high-fat diet-induced inflammation through regulation of lipid metabolism and suppression of NFκB activation in juvenile black seabream (Acanthopagrus schlegelii). J. Nutr. Sci. 2019, 8, e38.
- 23.Rajabi, A.A.; Castro, G.S.F.; Silva, R.P.D.; Nelson, R.C.; Thiesen, A.; Vannucchi, H.; Vine, D.F.; Proctor, S.D.; Field, C.J.; Curtis, J.M.; et al. Choline supplementation protects against liver damage by normalizing cholesterol metabolism in Pemt/Ldlr knockout mice fed a high-fat diet. J. Nutr. 2014, 144, 252–257.
- 24.Poulsen, E.T.; Pedersen, K.W.; Marzeda, A.M.; Enghild, J.J. Serum amyloid P component (SAP) interactome in human plasma containing physiological calcium levels. Biochemistry 2017, 56, 896–902.
- 25.Fernstrom, J.D.; Fernstrom, M.H. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain2. J. Nutr. 2007, 137, 1539S–1547S.
- 26.Murín, R.; Mohammadi, G.; Leibfritz, D.; Hamprecht, B. Glial metabolism of valine. Neurochem. Res. 2009, 34, 1195–1203.
- 27.Mahdy, A.M.; Webster, N.R. Histamine and antihistamines. Anaesth. Intensive Care Med. 2011, 12, 324–329.
- 28.Rao, K.V.R.; Reddy, P.V.B.; Tong, X.; Norenberg, M.D. Brain edema in acute liver failure: Inhibition by L-histidine. Am. J. Pathol. 2010, 176, 1400–1408.
- 29.Falany, C.N.; Johnson, M.R.; Barnes, S.; Diasio, R.B. Glycine and taurine conjugation of bile acids by a single enzyme. Molecular cloning and expression of human liver bile acid CoA: Amino acid N-acyltransferase. J. Biol. Chem. 1994, 269, 19375–19379.
- 30.Schaffer, S.W.; Jong, C.J.; Ramila, K.C.; Azuma, J. Physiological roles of taurine in heart and muscle. J. Biomed. Sci. 2010, 17, S2.
- 31.Ansari, F.A.; Ali, S.N.; Mahmood, R. Taurine mitigates nitrite-induced methemoglobin formation and oxidative damage in human erythrocytes. Environ. Sci. Pollut. Res. Int. 2017, 24, 19086–19097.
- 32.Ding, L.; Chang, M.; Guo, Y.; Zhang, L.; Xue, C.; Yanagita, T.; Zhang, T.; Wang, Y. Trimethylamine-N-oxide (TMAO)-induced atherosclerosis is associated with bile acid metabolism. Lipids Health Dis. 2018, 17, 286.
How to Cite
Zhu, Y.; Xie, S.; Shu, H.; Lu, Y.; Guo, J.; Li, Q.; Zhang, Y.; Yang, Y. Comparative Study on Metabolic Regulation of SAP Deletion in Normal Mice and Atherosclerotic Mice. Health and Metabolism 2025, 2 (3), 5. https://doi.org/10.53941/hm.2025.100020.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


