Aging is a complex and multifactorial process, characterized by a gradual decline of multiple organ systems. Increasing evidence suggests that organ crosstalk plays a crucial role in aging. It is particularly important in the development of age-related diseases like sarcopenia. The liver significantly impacts skeletal muscle health by influencing metabolic health, inflammatory signals, and the secretion of hepatokines. Chronic liver diseases, including non-alcoholic fatty liver disease (NAFLD), cirrhosis, and hepatocellular carcinoma (HCC), exacerbate sarcopenia by disruptiong the liver-muscle interactions. Recent studies have demonstrated that liver-derived metabolites, including ketone bodies, can modulate the skeletal muscle function. Notably, beta-hydroxybutyrate (BHB), a key liver-derived metabolite, has been shown to mediate post-translational modifications (PTMs) in muscle, reversing sarcopenia through beta-hydroxybutyrylation. This review explores the relationship between liver aging, chronic liver diseases, and sarcopenia. It focuses on mediators of liver-muscle crosstalk, including metabolic integration, hepatokines, and miRNAs in extracellular vesicles (EVs). We highlight the impact of liver-derived metabolites on skeletal muscle post-translational modifications, particularly the role of BHB in muscle rejuvenation and sarcopenia reversal. Understanding these mechanisms provides new insights into potential therapeutic strategies for mitigating sarcopenia via living aging intervention.
- Open Access
- Review
From Liver to Muscle: Crosstalk Mechanisms and Interventions in Sarcopenia
- Qiquan Wang 1, †,
- Meng Yao 1, †,
- Xiang Wang 1, †,
- Xinqiang Lan 1,
- Gailing Fan 2,
- Yang Xiang 1, *
Author Information
Received: 27 Mar 2025 | Revised: 25 Apr 2025 | Accepted: 23 May 2025 | Published: 17 Jul 2025
Abstract
Graphical Abstract
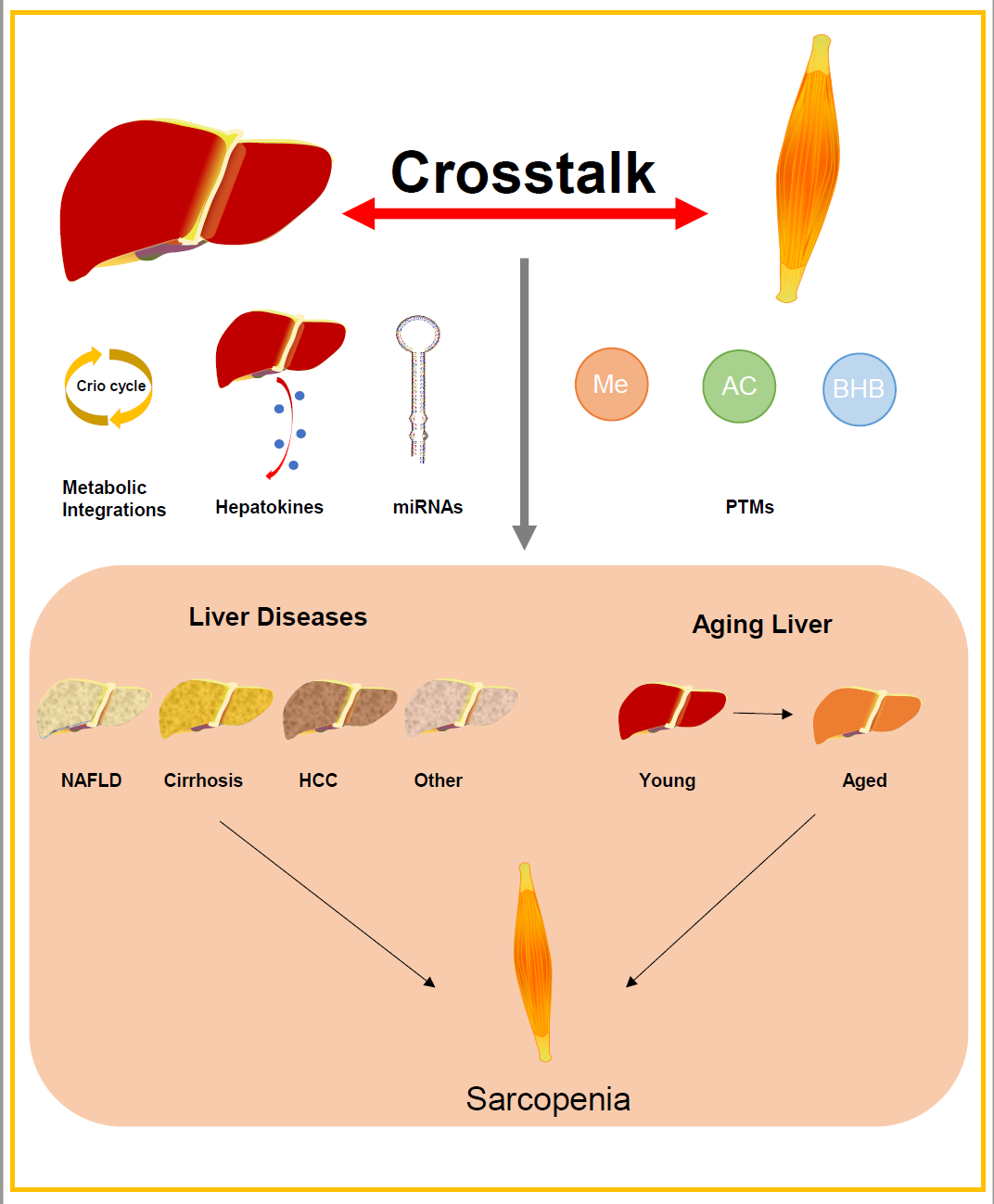
Keywords
skeletal muscle aging | liver aging | sarcopenia | organ crosstalk | liver-muscle axis | chronic liver diseases | hepatokines
References
- 1.López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278.
- 2.Nie, C.; Li, Y.; Li, R.; Yan, Y.; Zhang, D.; Li, T.; Li, Z.; Sun, Y.; Zhen, H.; Ding, J.; et al. Distinct biological ages of organs and systems identified from a multi-omics study. Cell Rep. 2022, 38, 110459. https://doi.org/10.1016/j.celrep.2022.110459.
- 3.Tokizane, K.; Imai, S.I. Inter-organ communication is a critical machinery to regulate metabolism and aging. Trends Endocrinol. Metab. 2024. https://doi.org/10.1016/j.tem.2024.11.013.
- 4.Wang, S.; Liu, Y.; Chen, J.; He, Y.; Ma, W.; Liu, X.; Sun, X. Effects of multi-organ crosstalk on the physiology and pathology of adipose tissue. Front. Endocrinol. 2023, 14, 1198984. https://doi.org/10.3389/fendo.2023.1198984.
- 5.Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota-gut-brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target. Ther. 2024, 9, 37. https://doi.org/10.1038/s41392-024-01743-1.
- 6.Sheng, R.; Cao, M.; Song, M.; Wang, M.; Zhang, Y.; Shi, L.; Xie, T.; Li, Y.; Wang, J.; Rui, Y. Muscle-bone crosstalk via endocrine signals and potential targets for osteosarcopenia-related fracture. J. Orthop. Transl. 2023, 43, 36–46. https://doi.org/10.1016/j.jot.2023.09.007.
- 7.Oishi, Y.; Manabe, I. Organ System Crosstalk in Cardiometabolic Disease in the Age of Multimorbidity. Front. Cardiovasc. Med. 2020, 7, 64. https://doi.org/10.3389/fcvm.2020.00064.
- 8.Curaj, A.; Vanholder, R.; Loscalzo, J.; Quach, K.; Wu, Z.; Jankowski, V.; Jankowski, J. Cardiovascular Consequences of Uremic Metabolites: An Overview of the Involved Signaling Pathways. Circ. Res. 2024, 134, 592–613. https://doi.org/10.1161/circresaha.123.324001.
- 9.Alcalde-Estévez, E.; Sosa, P.; Asenjo-Bueno, A.; Plaza, P.; Olmos, G.; Naves-Díaz, M.; Rodríguez-Puyol, D.; López-Ongil, S.; Ruiz-Torres, M.P. Uraemic toxins impair skeletal muscle regeneration by inhibiting myoblast proliferation, reducing myogenic differentiation, and promoting muscular fibrosis. Sci. Rep. 2021, 11, 512. https://doi.org/10.1038/s41598-020-79186-1.
- 10.Huang, N.; Ge, M.; Liu, X.; Tian, X.; Yin, P.; Bao, Z.; Cao, F.; Shyh-Chang, N.; Dong, B.; Dai, L.; et al. A framework of biomarkers for skeletal muscle aging: A consensus statement by the Aging Biomarker Consortium. Life Med. 2024, 3, lnaf001. https://doi.org/10.1093/lifemedi/lnaf001.
- 11.Deschenes, M.R. Effects of aging on muscle fibre type and size. Sports Med. 2004, 34, 809–824. https://doi.org/10.2165/00007256-200434120-00002.
- 12.Marcus, R.L.; Addison, O.; Kidde, J.P.; Dibble, L.E.; Lastayo, P.C. Skeletal muscle fat infiltration: Impact of age, inactivity, and exercise. J. Nutr. Health Aging 2010, 14, 362–366. https://doi.org/10.1007/s12603-010-0081-2.
- 13.Alnaqeeb, M.A.; Al Zaid, N.S.; Goldspink, G. Connective tissue changes and physical properties of developing and ageing skeletal muscle. J. Anat. 1984, 139, 677–689.
- 14.Wang, S.; Yu, G.; Xie, L. Asymmetric Cell Division and Satellite Cell Fate Regulation in Skeletal Muscle Aging and Disease. Health Metab. 2024, 1, 5. https://doi.org/10.53941/hm.2024.100005.
- 15.Kedlian, V.R.; Wang, Y.; Liu, T.; Chen, X.; Bolt, L.; Tudor, C.; Shen, Z.; Fasouli, E.S.; Prigmore, E.; Kleshchevnikov, V.; et al. Human skeletal muscle aging atlas. Nat. Aging 2024, 4, 727–744. https://doi.org/10.1038/s43587-024-00613-3.
- 16.Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127, 990s–991s. https://doi.org/10.1093/jn/127.5.990S.
- 17.Kirk, B.; Cawthon, P.M.; Arai, H.; Ávila-Funes, J.A.; Barazzoni, R.; Bhasin, S.; Binder, E.F.; Bruyere, O.; Cederholm, T.; Chen, L.K.; et al. The Conceptual Definition of Sarcopenia: Delphi Consensus from the Global Leadership Initiative in Sarcopenia (GLIS). Age Ageing 2024, 53, afae052. https://doi.org/10.1093/ageing/afae052.
- 18.Ren, J.; Song, M.; Zhang, W.; Cai, J.P.; Cao, F.; Cao, Z.; Chan, P.; Chen, C.; Chen, G.; Chen, H.Z.; et al. The Aging Biomarker Consortium represents a new era for aging research in China. Nat. Med. 2023, 29, 2162–2165. https://doi.org/10.1038/s41591-023-02444-y.
- 19.Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical definition of sarcopenia. Clin. Cases Miner. Bone Metab. 2014, 11, 177–180.
- 20.Cho, M.R.; Lee, S.; Song, S.K. A Review of Sarcopenia Pathophysiology, Diagnosis, Treatment and Future Direction. J. Korean Med. Sci. 2022, 37, e146. https://doi.org/10.3346/jkms.2022.37.e146.
- 21.Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. https://doi.org/10.1093/ageing/afz046.
- 22.Kurosawa, T.; Goto, M.; Kaji, N.; Aikiyo, S.; Mihara, T.; Ikemoto-Uezumi, M.; Toyoda, M.; Kanazawa, N.; Nakazawa, T.; Hori, M.; et al. Liver fibrosis-induced muscle atrophy is mediated by elevated levels of circulating TNFα. Cell Death Dis. 2021, 12, 11. https://doi.org/10.1038/s41419-020-03353-5.
- 23.Montano-Loza, A.J.; Meza-Junco, J.; Prado, C.M.; Lieffers, J.R.; Baracos, V.E.; Bain, V.G.; Sawyer, M.B. Muscle wasting is associated with mortality in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2012, 10, 166–173. https://doi.org/10.1016/j.cgh.2011.08.028.
- 24.Vandervoort, A.A. Aging of the human neuromuscular system. Muscle Nerve 2001, 25, 17–25. https://doi.org/10.1002/mus.1215.
- 25.Isoyama, N.; Qureshi, A.R.; Avesani, C.M.; Lindholm, B.; Bàràny, P.; Heimbürger, O.; Cederholm, T.; Stenvinkel, P.; Carrero, J.J. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1720–1728. https://doi.org/10.2215/cjn.10261013.
- 26.Ji, T.; Li, Y.; Ma, L. Sarcopenic Obesity: An Emerging Public Health Problem. Aging Dis. 2022, 13, 379–388. https://doi.org/10.14336/ad.2021.1006.
- 27.Trierweiler, H.; Kisielewicz, G.; Hoffmann Jonasson, T.; Rasmussen Petterle, R.; Aguiar Moreira, C.; Zeghbi Cochenski Borba, V. Sarcopenia: A chronic complication of type 2 diabetes mellitus. Diabetol. Metab. Syndr. 2018, 10, 25. https://doi.org/10.1186/s13098-018-0326-5.
- 28.Curcio, F.; Testa, G.; Liguori, I.; Papillo, M.; Flocco, V.; Panicara, V.; Galizia, G.; Della-Morte, D.; Gargiulo, G.; Cacciatore, F.; et al. Sarcopenia and Heart Failure. Nutrients 2020, 12, 211. https://doi.org/10.3390/nu12010211.
- 29.Anjanappa, M.; Corden, M.; Green, A.; Roberts, D.; Hoskin, P.; McWilliam, A.; Choudhury, A. Sarcopenia in cancer: Risking more than muscle loss. Tech. Innov. Patient Support. Radiat. Oncol. 2020, 16, 50–57. https://doi.org/10.1016/j.tipsro.2020.10.001.
- 30.Brooks, G.A. Lactate as a fulcrum of metabolism. Redox Biol. 2020, 35, 101454. https://doi.org/10.1016/j.redox.2020.101454.
- 31.Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. https://doi.org/10.3390/nu10111564.
- 32.Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. https://doi.org/10.1038/nrendo.2012.49.
- 33.Fisher, F.M.; Maratos-Flier, E. Understanding the Physiology of FGF21. Annu. Rev. Physiol. 2016, 78, 223–241. https://doi.org/10.1146/annurev-physiol-021115-105339.
- 34.Yeo, Y.H.; Lai, Y.C. Redox Regulation of Metabolic Syndrome: Recent Developments in Skeletal Muscle Insulin Resistance and Non-alcoholic Fatty Liver Disease (NAFLD). Curr. Opin. Physiol. 2019, 9, 79–86. https://doi.org/10.1016/j.cophys.2019.05.003.
- 35.Fan, L.; Sweet, D.R.; Prosdocimo, D.A.; Vinayachandran, V.; Chan, E.R.; Zhang, R.; Ilkayeva, O.; Lu, Y.; Keerthy, K.S.; Booth, C.E.; et al. Muscle Krüppel-like factor 15 regulates lipid flux and systemic metabolic homeostasis. J. Clin. Investig. 2021, 131. https://doi.org/10.1172/jci139496.
- 36.Darkwah, S.; Park, E.J.; Myint, P.K.; Ito, A.; Appiah, M.G.; Obeng, G.; Kawamoto, E.; Shimaoka, M. Potential Roles of Muscle-Derived Extracellular Vesicles in Remodeling Cellular Microenvironment: Proposed Implications of the Exercise-Induced Myokine, Irisin. Front. Cell Dev. Biol. 2021, 9, 634853. https://doi.org/10.3389/fcell.2021.634853.
- 37.Keating, S.E.; Hackett, D.A.; Parker, H.M.; O’Connor, H.T.; Gerofi, J.A.; Sainsbury, A.; Baker, M.K.; Chuter, V.H.; Caterson, I.D.; George, J.; et al. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J. Hepatol. 2015, 63, 174–182. https://doi.org/10.1016/j.jhep.2015.02.022.
- 38.Mero, A. Leucine supplementation and intensive training. Sports Med. 1999, 27, 347–358. https://doi.org/10.2165/00007256-199927060-00001.
- 39.Campanari, D.D.; Cipriano, U.G.; Fraga-Silva, T.F.C.; Ramalho, L.N.Z.; Ovidio, P.P.; Jordão Júnior, A.A.; Bonato, V.L.D.; Ferriolli, E. Effect of Dietary Supplementation with Omega-3 Fatty Acid on the Generation of Regulatory T Lymphocytes and on Antioxidant Parameters and Markers of Oxidative Stress in the Liver Tissue of IL-10 Knockout Mice. Nutrients 2024, 16, 634. https://doi.org/10.3390/nu16050634.
- 40.Deng, Y.; Hu, M.; Huang, S.; Fu, N. Molecular mechanism and therapeutic significance of essential amino acids in metabolically associated fatty liver disease. J. Nutr. Biochem. 2024, 126, 109581. https://doi.org/10.1016/j.jnutbio.2024.109581.
- 41.Jeromson, S.; Gallagher, I.J.; Galloway, S.D.; Hamilton, D.L. Omega-3 Fatty Acids and Skeletal Muscle Health. Mar. Drugs 2015, 13, 6977–7004. https://doi.org/10.3390/md13116977.
- 42.Hong, H.C.; Hwang, S.Y.; Choi, H.Y.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; Choi, D.S.; Choi, K.M. Relationship between sarcopenia and nonalcoholic fatty liver disease: The Korean Sarcopenic Obesity Study. Hepatology 2014, 59, 1772–1778. https://doi.org/10.1002/hep.26716.
- 43.Allen, S.L.; Quinlan, J.I.; Dhaliwal, A.; Armstrong, M.J.; Elsharkawy, A.M.; Greig, C.A.; Lord, J.M.; Lavery, G.G.; Breen, L. Sarcopenia in chronic liver disease: Mechanisms and countermeasures. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G241–g257. https://doi.org/10.1152/ajpgi.00373.2020.
- 44.Tantai, X.; Liu, Y.; Yeo, Y.H.; Praktiknjo, M.; Mauro, E.; Hamaguchi, Y.; Engelmann, C.; Zhang, P.; Jeong, J.Y.; van Vugt, J.L.A.; et al. Effect of sarcopenia on survival in patients with cirrhosis: A meta-analysis. J. Hepatol. 2022, 76, 588–599. https://doi.org/10.1016/j.jhep.2021.11.006.
- 45.Bhanji, R.A.; Narayanan, P.; Allen, A.M.; Malhi, H.; Watt, K.D. Sarcopenia in hiding: The risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis. Hepatology 2017, 66, 2055–2065. https://doi.org/10.1002/hep.29420.
- 46.Issa, D.; Alkhouri, N.; Tsien, C.; Shah, S.; Lopez, R.; McCullough, A.; Dasarathy, S. Presence of sarcopenia (muscle wasting) in patients with nonalcoholic steatohepatitis. Hepatology 2014, 60, 428–429. https://doi.org/10.1002/hep.26908.
- 47.Lee, Y.H.; Kim, S.U.; Song, K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Lee, B.W.; Kang, E.S.; Cha, B.S.; Han, K.H. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: Nationwide surveys (KNHANES 2008-2011). Hepatology 2016, 63, 776–786. https://doi.org/10.1002/hep.28376.
- 48.Petta, S.; Ciminnisi, S.; Di Marco, V.; Cabibi, D.; Cammà, C.; Licata, A.; Marchesini, G.; Craxì, A. Sarcopenia is associated with severe liver fibrosis in patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2017, 45, 510–518. https://doi.org/10.1111/apt.13889.
- 49.Guo, W.; Zhao, X.; Miao, M.; Liang, X.; Li, X.; Qin, P.; Lu, J.; Zhu, W.; Wu, J.; Zhu, C.; et al. Association Between Skeletal Muscle Mass and Severity of Steatosis and Fibrosis in Non-alcoholic Fatty Liver Disease. Front. Nutr. 2022, 9, 883015. https://doi.org/10.3389/fnut.2022.883015.
- 50.Chen, M.; Liu, J.; Xia, X.; Wang, Y.; Zheng, H. Causal relationship between non-alcoholic fatty liver disease and sarcopenia: A bidirectional Mendelian randomization study. Front. Med. 2024, 11, 1422499. https://doi.org/10.3389/fmed.2024.1422499.
- 51.Harris, S.E.; Poolman, T.M.; Arvaniti, A.; Cox, R.D.; Gathercole, L.L.; Tomlinson, J.W. The American lifestyle-induced obesity syndrome diet in male and female rodents recapitulates the clinical and transcriptomic features of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G345–g360. https://doi.org/10.1152/ajpgi.00055.2020.
- 52.EASL Clinical Practice Guidelines: Management of cholestatic liver diseases. J. Hepatol. 2009, 51, 237–267. https://doi.org/10.1016/j.jhep.2009.04.009.
- 53.EASL Clinical Practice Guidelines on acute-on-chronic liver failure. J. Hepatol. 2023, 79, 461–491. https://doi.org/10.1016/j.jhep.2023.04.021.
- 54.Abdelbasset, W.K.; Nambi, G.; Elsayed, S.H.; Moawd, S.A.; Ibrahim, A.A.; Verma, A.; Tantawy, S.A.; Kamel, D.M.; Saleh, A.K.; Aldhafian, O.R.; et al. Prevalence and Nonpharmacological Interventions for Sarcopenia among Cirrhotic Patients. Dis. Markers 2021, 2021, 8866093. https://doi.org/10.1155/2021/8866093.
- 55.Aamann, L.; Dam, G.; Borre, M.; Drljevic-Nielsen, A.; Overgaard, K.; Andersen, H.; Vilstrup, H.; Aagaard, N.K. Resistance Training Increases Muscle Strength and Muscle Size in Patients With Liver Cirrhosis. Clin. Gastroenterol. Hepatol. 2020, 18, 1179–1187.e1176. https://doi.org/10.1016/j.cgh.2019.07.058.
- 56.Anand, A.C. Nutrition and Muscle in Cirrhosis. J. Clin. Exp. Hepatol. 2017, 7, 340–357. https://doi.org/10.1016/j.jceh.2017.11.001.
- 57.Anand, A.; Nambirajan, A.; Kumar, V.; Agarwal, S.; Sharma, S.; Mohta, S.; Gopi, S.; Kaushal, K.; Gunjan, D.; Singh, N.; et al. Alterations in Autophagy and Mammalian Target of Rapamycin (mTOR) Pathways Mediate Sarcopenia in Patients with Cirrhosis. J. Clin. Exp. Hepatol. 2022, 12, 510–518. https://doi.org/10.1016/j.jceh.2021.05.004.
- 58.Alexopoulos, T.; Vasilieva, L.; Kontogianni, M.D.; Tenta, R.; Georgiou, A.; Stroumpouli, E.; Mani, I.; Alexopoulou, A. Myostatin in combination with creatine phosphokinase or albumin may differentiate patients with cirrhosis and sarcopenia. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, G543–g551. https://doi.org/10.1152/ajpgi.00184.2021.
- 59.Abuelazm, M.; Fares, A.; Elhady, M.M.; Amin, A.M.; Khan, U.; Gowaily, I.; Jaber, F. Branched-Chain Amino Acid Supplements for Sarcopenia in Liver Cirrhosis: A Systematic Review and Meta-analysis. J. Clin. Exp. Hepatol. 2025, 15, 102417. https://doi.org/10.1016/j.jceh.2024.102417.
- 60.Artru, F.; Miquet, X.; Azahaf, M.; Labreuche, J.; Ntandja Wandji, L.C.; Sergent, G.; Nobécourt, A.; Toumelin, P.; Lassailly, G.; Dharancy, S.; et al. Consequences of TIPSS placement on the body composition of patients with cirrhosis and severe portal hypertension: A large retrospective CT-based surveillance. Aliment. Pharmacol. Ther. 2020, 52, 1516–1526. https://doi.org/10.1111/apt.16080.
- 61.Harimoto, N.; Shirabe, K.; Yamashita, Y.I.; Ikegami, T.; Yoshizumi, T.; Soejima, Y.; Ikeda, T.; Maehara, Y.; Nishie, A.; Yamanaka, T. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br. J. Surg. 2013, 100, 1523–1530. https://doi.org/10.1002/bjs.9258.
- 62.Akce, M.; Liu, Y.; Zakka, K.; Martini, D.J.; Draper, A.; Alese, O.B.; Shaib, W.L.; Wu, C.; Wedd, J.P.; Sellers, M.T.; et al. Impact of Sarcopenia, BMI, and Inflammatory Biomarkers on Survival in Advanced Hepatocellular Carcinoma Treated With Anti-PD-1 Antibody. Am. J. Clin. Oncol. 2021, 44, 74–81. https://doi.org/10.1097/coc.0000000000000787.
- 63.Chang, K.V.; Chen, J.D.; Wu, W.T.; Huang, K.C.; Hsu, C.T.; Han, D.S. Association between Loss of Skeletal Muscle Mass and Mortality and Tumor Recurrence in Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Liver Cancer 2018, 7, 90–103. https://doi.org/10.1159/000484950.
- 64.Bai, Y.; Liu, J.; Wang, Y.; Zhou, B.; Liu, X.; Dong, X.; Zheng, C. Impact of Sarcopenia on Prognosis in Primary Hepatocellular Carcinoma Patients Treated with Transcatheter Arterial Chemoembolization: A Single Center Retrospective Study. J. Cancer 2024, 15, 1837–1847. https://doi.org/10.7150/jca.92976.
- 65.Cao, J.; Huang, Y.; Zhu, M.; Wang, Z.; Jin, Z.; Xiong, Z. Causal association of sarcopenia with hepatocellular carcinoma risk in European population: A Mendelian randomization study. Front. Nutr. 2024, 11, 1292834. https://doi.org/10.3389/fnut.2024.1292834.
- 66.Erdem, M.; Möckel, D.; Jumpertz, S.; John, C.; Fragoulis, A.; Rudolph, I.; Wulfmeier, J.; Springer, J.; Horn, H.; Koch, M.; et al. Macrophages protect against loss of adipose tissue during cancer cachexia. J. Cachexia Sarcopenia Muscle 2019, 10, 1128–1142. https://doi.org/10.1002/jcsm.12450.
- 67.Osna, N.A.; Donohue, T.M., Jr.; Kharbanda, K.K. Alcoholic Liver Disease: Pathogenesis and Current Management. Alcohol. Res. 2017, 38, 147–161.
- 68.Dasarathy, J.; McCullough, A.J.; Dasarathy, S. Sarcopenia in Alcoholic Liver Disease: Clinical and Molecular Advances. Alcohol. Clin. Exp. Res. 2017, 41, 1419–1431. https://doi.org/10.1111/acer.13425.
- 69.Boster, J.M.; Goodrich, N.P.; Spino, C.; Loomes, K.M.; Alonso, E.M.; Kamath, B.M.; Sokol, R.J.; Karpen, S.; Miethke, A.; Shneider, B.L.; et al. Sarcopenia is associated with osteopenia and impaired quality of life in children with genetic intrahepatic cholestatic liver disease. Hepatol. Commun. 2023, 7. https://doi.org/10.1097/hc9.0000000000000293.
- 70.Krawitt, E.L. Clinical features and management of autoimmune hepatitis. World J. Gastroenterol. 2008, 14, 3301–3305. https://doi.org/10.3748/wjg.14.3301.
- 71.Amevor, A.A.; Yodoshi, T.; Trout, A.T.; Dillman, J.R.; Singh, R.; Jarvis, R.; Fei, L.; Liu, C.; Taylor, A.; Miethke, A.; et al. Sarcopenia is highly prevalent in children with autoimmune liver diseases and is linked to visceral fat and parent-perceived general health. Liver Int. 2022, 42, 394–401. https://doi.org/10.1111/liv.15108.
- 72.Kaido, T.; Ogawa, K.; Fujimoto, Y.; Ogura, Y.; Hata, K.; Ito, T.; Tomiyama, K.; Yagi, S.; Mori, A.; Uemoto, S. Impact of sarcopenia on survival in patients undergoing living donor liver transplantation. Am. J. Transplant. 2013, 13, 1549–1556. https://doi.org/10.1111/ajt.12221.
- 73.Markakis, G.E.; Lai, J.C.; Karakousis, N.D.; Papatheodoridis, G.V.; Psaltopoulou, T.; Merli, M.; Sergentanis, T.N.; Cholongitas, E. Sarcopenia As a Predictor of Survival and Complications of Patients With Cirrhosis After Liver Transplantation: A Systematic Review and Meta-Analysis. Clin. Transplant. 2025, 39, e70088. https://doi.org/10.1111/ctr.70088.
- 74.Wang, Q.; Lan, X.; Ke, H.; Xu, S.; Huang, C.; Wang, J.; Wang, X.; Huang, T.; Wu, X.; Chen, M.; et al. Histone β-hydroxybutyrylation is critical in reversal of sarcopenia. Aging Cell 2024, 23, e14284. https://doi.org/10.1111/acel.14284.
- 75.Jiang, M.; Zheng, Z.; Wang, X.; Chen, Y.; Qu, J.; Ding, Q.; Zhang, W.; Liu, Y.S.; Yang, J.; Tang, W.; et al. A biomarker framework for liver aging: The Aging Biomarker Consortium consensus statement. Life Med. 2024, 3, lnae004. https://doi.org/10.1093/lifemedi/lnae004.
- 76.Heinke, P.; Rost, F.; Rode, J.; Trus, P.; Simonova, I.; Lázár, E.; Feddema, J.; Welsch, T.; Alkass, K.; Salehpour, M.; et al. Diploid hepatocytes drive physiological liver renewal in adult humans. Cell Syst. 2022, 13, 499–507.e412. https://doi.org/10.1016/j.cels.2022.05.001.
- 77.Buzzelli, G.; Cotrozzi, G.; Relli, P.; Matassi, L.; Simondi, P.; Orioli, S.; Bandini, P.; Giovacchini, L.; Lupi, R.; Gentilini, P. [Prevalence of glucose intolerance and diabetes in chronic liver disease. I. Influence of familial diabetes and aging]. Boll. Soc. Ital. Biol. Sper. 1985, 61, 1439–1444.
- 78.Nakajima, T.; Nakashima, T.; Yamaoka, J.; Shibuya, A.; Konishi, E.; Okada, Y.; Jo, M.; Nishikawa, T.; Itoh, Y.; Yoshikawa, T. Greater age and hepatocellular aging are independent risk factors for hepatocellular carcinoma arising from non-B non-C non-alcoholic chronic liver disease. Pathol. Int. 2011, 61, 572–576. https://doi.org/10.1111/j.1440-1827.2011.02743.x.
- 79.Sundaram, V.; Jalan, R.; Shah, P.; Singal, A.K.; Patel, A.A.; Wu, T.; Noureddin, M.; Mahmud, N.; Wong, R.J. Acute on Chronic Liver Failure From Nonalcoholic Fatty Liver Disease: A Growing and Aging Cohort With Rising Mortality. Hepatology 2021, 73, 1932–1944. https://doi.org/10.1002/hep.31566.
- 80.Penrice, D.D.; Jalan-Sakrikar, N.; Jurk, D.; Passos, J.F.; Simonetto, D.A. Telomere dysfunction in chronic liver disease: The link from aging. Hepatology 2024, 80, 951–964. https://doi.org/10.1097/hep.0000000000000426.
- 81.Maeso-Díaz, R.; Ortega-Ribera, M.; Lafoz, E.; Lozano, J.J.; Baiges, A.; Francés, R.; Albillos, A.; Peralta, C.; García-Pagán, J.C.; Bosch, J.; et al. Aging Influences Hepatic Microvascular Biology and Liver Fibrosis in Advanced Chronic Liver Disease. Aging Dis. 2019, 10, 684–698. https://doi.org/10.14336/ad.2019.0127.
- 82.Meng, S.S.; Gu, H.W.; Zhang, T.; Li, Y.S.; Tang, H.B. Gradual deterioration of fatty liver disease to liver cancer via inhibition of AMPK signaling pathways involved in energy-dependent disorders, cellular aging, and chronic inflammation. Front. Oncol. 2023, 13, 1099624. https://doi.org/10.3389/fonc.2023.1099624.
- 83.Aravinthan, A.D.; Alexander, G.J.M. Senescence in chronic liver disease: Is the future in aging? J. Hepatol. 2016, 65, 825–834. https://doi.org/10.1016/j.jhep.2016.05.030.
- 84.Wan, Y.; Li, X.; Slevin, E.; Harrison, K.; Li, T.; Zhang, Y.; Klaunig, J.E.; Wu, C.; Shetty, A.K.; Dong, X.C.; et al. Endothelial dysfunction in pathological processes of chronic liver disease during aging. Faseb J. 2022, 36, e22125. https://doi.org/10.1096/fj.202101426R.
- 85.Oh, H.S.; Rutledge, J.; Nachun, D.; Pálovics, R.; Abiose, O.; Moran-Losada, P.; Channappa, D.; Urey, D.Y.; Kim, K.; Sung, Y.J.; et al. Organ aging signatures in the plasma proteome track health and disease. Nature 2023, 624, 164–172. https://doi.org/10.1038/s41586-023-06802-1.
- 86.Xu, J.; Xu, Z.X.; Yang, Q.F.; Zhuang, J.; Zhu, X.; Yao, J. Association between the sarcopenia index and abnormal liver function in the adult population in the United States: A cross-sectional study. Front. Med. 2023, 10, 1266253. https://doi.org/10.3389/fmed.2023.1266253.
- 87.Schmucker, D.L. Age-related changes in liver structure and function: Implications for disease ? Exp. Gerontol. 2005, 40, 650–659. https://doi.org/10.1016/j.exger.2005.06.009.
- 88.Khosla, S.; Farr, J.N.; Tchkonia, T.; Kirkland, J.L. The role of cellular senescence in ageing and endocrine disease. Nat. Rev. Endocrinol. 2020, 16, 263–275. https://doi.org/10.1038/s41574-020-0335-y.
- 89.Timchenko, N.A. Aging and liver regeneration. Trends Endocrinol. Metab. 2009, 20, 171–176. https://doi.org/10.1016/j.tem.2009.01.005.
- 90.Janssen, I.; Heymsfield, S.B.; Wang, Z.M.; Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J. Appl. Physiol. 2000, 89, 81–88. https://doi.org/10.1152/jappl.2000.89.1.81.
- 91.Wolfe, R.R. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 2006, 84, 475–482. https://doi.org/10.1093/ajcn/84.3.475.
- 92.Addison, O.; Marcus, R.L.; Lastayo, P.C.; Ryan, A.S. Intermuscular fat: A review of the consequences and causes. Int. J. Endocrinol. 2014, 2014, 309570. https://doi.org/10.1155/2014/309570.
- 93.Bian, A.; Ma, Y.; Zhou, X.; Guo, Y.; Wang, W.; Zhang, Y.; Wang, X. Association between sarcopenia and levels of growth hormone and insulin-like growth factor-1 in the elderly. BMC Musculoskelet. Disord. 2020, 21, 214. https://doi.org/10.1186/s12891-020-03236-y.
- 94.Uemura, K.; Doi, T.; Lee, S.; Shimada, H. Sarcopenia and Low Serum Albumin Level Synergistically Increase the Risk of Incident Disability in Older Adults. J. Am. Med. Dir. Assoc. 2019, 20, 90–93. https://doi.org/10.1016/j.jamda.2018.06.011.
- 95.Bano, G.; Trevisan, C.; Carraro, S.; Solmi, M.; Luchini, C.; Stubbs, B.; Manzato, E.; Sergi, G.; Veronese, N. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas 2017, 96, 10–15. https://doi.org/10.1016/j.maturitas.2016.11.006.
- 96.Jiang, H.; Li, L.; Zhang, X.; He, J.; Chen, C.; Sun, R.; Chen, Y.; Xia, L.; Wen, L.; Chen, Y.; et al. Novel insights into the association between genetically proxied inhibition of proprotein convertase subtilisin/kexin type 9 and risk of sarcopenia. J. Cachexia Sarcopenia Muscle 2024, 15, 2417–2425. https://doi.org/10.1002/jcsm.13575.
- 97.Chen, J.L.; Chen, D.M.; Luo, C.; Sun, Y.; Zhao, Y.X.; Huang, C.Q.; Zhao, K.X.; Xiao, Q. Fibrinogen, fibrin degradation products and risk of sarcopenia. Clin. Nutr. 2021, 40, 4830–4837. https://doi.org/10.1016/j.clnu.2021.06.031.
- 98.Stefan, N.; Häring, H.U. The role of hepatokines in metabolism. Nat. Rev. Endocrinol. 2013, 9, 144–152. https://doi.org/10.1038/nrendo.2012.258.
- 99.Tipton, K.D.; Wolfe, R.R. Exercise, protein metabolism, and muscle growth. Int. J. Sport. Nutr. Exerc. Metab. 2001, 11, 109–132. https://doi.org/10.1123/ijsnem.11.1.109.
- 100.Kumar, K.G.; Trevaskis, J.L.; Lam, D.D.; Sutton, G.M.; Koza, R.A.; Chouljenko, V.N.; Kousoulas, K.G.; Rogers, P.M.; Kesterson, R.A.; Thearle, M.; et al. Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab. 2008, 8, 468–481. https://doi.org/10.1016/j.cmet.2008.10.011.
- 101.Butler, A.A.; Tam, C.S.; Stanhope, K.L.; Wolfe, B.M.; Ali, M.R.; O’Keeffe, M.; St-Onge, M.P.; Ravussin, E.; Havel, P.J. Low circulating adropin concentrations with obesity and aging correlate with risk factors for metabolic disease and increase after gastric bypass surgery in humans. J. Clin. Endocrinol. Metab. 2012, 97, 3783–3791. https://doi.org/10.1210/jc.2012-2194.
- 102.Yu, X.; Burgess, S.C.; Ge, H.; Wong, K.K.; Nassem, R.H.; Garry, D.J.; Sherry, A.D.; Malloy, C.R.; Berger, J.P.; Li, C. Inhibition of cardiac lipoprotein utilization by transgenic overexpression of Angptl4 in the heart. Proc. Natl. Acad. Sci. USA 2005, 102, 1767–1772. https://doi.org/10.1073/pnas.0409564102.
- 103.McCulloch, L.J.; Bramwell, L.R.; Knight, B.; Kos, K. Circulating and tissue specific transcription of angiopoietin-like protein 4 in human Type 2 diabetes. Metabolism 2020, 106, 154192. https://doi.org/10.1016/j.metabol.2020.154192.
- 104.Pal, D.; Dasgupta, S.; Kundu, R.; Maitra, S.; Das, G.; Mukhopadhyay, S.; Ray, S.; Majumdar, S.S.; Bhattacharya, S. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat. Med. 2012, 18, 1279–1285. https://doi.org/10.1038/nm.2851.
- 105.Srinivas, P.R.; Wagner, A.S.; Reddy, L.V.; Deutsch, D.D.; Leon, M.A.; Goustin, A.S.; Grunberger, G. Serum alpha 2-HS-glycoprotein is an inhibitor of the human insulin receptor at the tyrosine kinase level. Mol. Endocrinol. 1993, 7, 1445–1455. https://doi.org/10.1210/mend.7.11.7906861.
- 106.Meex, R.C.; Hoy, A.J.; Morris, A.; Brown, R.D.; Lo, J.C.; Burke, M.; Goode, R.J.; Kingwell, B.A.; Kraakman, M.J.; Febbraio, M.A.; et al. Fetuin B Is a Secreted Hepatocyte Factor Linking Steatosis to Impaired Glucose Metabolism. Cell Metab. 2015, 22, 1078–1089. https://doi.org/10.1016/j.cmet.2015.09.023.
- 107.Li, Z.; Lin, M.; Liu, C.; Wang, D.; Shi, X.; Chen, Z.; Liu, Y.; Yang, S.; Li, X. Fetuin-B links nonalcoholic fatty liver disease to type 2 diabetes via inducing insulin resistance: Association and path analyses. Cytokine 2018, 108, 145–150. https://doi.org/10.1016/j.cyto.2018.03.023.
- 108.Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 2005, 115, 1627–1635. https://doi.org/10.1172/jci23606.
- 109.Oost, L.J.; Kustermann, M.; Armani, A.; Blaauw, B.; Romanello, V. Fibroblast growth factor 21 controls mitophagy and muscle mass. J. Cachexia Sarcopenia Muscle 2019, 10, 630–642. https://doi.org/10.1002/jcsm.12409.
- 110.Kim, C.S.; Joe, Y.; Choi, H.S.; Back, S.H.; Park, J.W.; Chung, H.T.; Roh, E.; Kim, M.S.; Ha, T.Y.; Yu, R. Deficiency of fibroblast growth factor 21 aggravates obesity-induced atrophic responses in skeletal muscle. J Inflamm (Lond) 2019, 16, 17. https://doi.org/10.1186/s12950-019-0221-3.
- 111.Jung, T.W.; Chung, Y.H.; Kim, H.C.; Abd El-Aty, A.M.; Jeong, J.H. Hyperlipidemia-induced hepassocin in the liver contributes to insulin resistance in skeletal muscle. Mol. Cell Endocrinol. 2018, 470, 26–33. https://doi.org/10.1016/j.mce.2017.10.014.
- 112.Cheng, K.P.; Ou, H.Y.; Hung, H.C.; Li, C.H.; Fan, K.C.; Wu, J.S.; Wu, H.T.; Chang, C.J. Unsaturated Fatty Acids Increase the Expression of Hepassocin through a Signal Transducer and Activator of Transcription 3-Dependent Pathway in HepG2 Cells. Lipids 2018, 53, 863–869. https://doi.org/10.1002/lipd.12099.
- 113.Hwang, H.J.; Jung, T.W.; Hong, H.C.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Choi, K.M.; Choi, D.S.; Baik, S.H.; Yoo, H.J. LECT2 induces atherosclerotic inflammatory reaction via CD209 receptor-mediated JNK phosphorylation in human endothelial cells. Metabolism 2015, 64, 1175–1182. https://doi.org/10.1016/j.metabol.2015.06.001.
- 114.Misu, H. Pathophysiological significance of hepatokine overproduction in type 2 diabetes. Diabetol. Int. 2018, 9, 224–233. https://doi.org/10.1007/s13340-018-0368-9.
- 115.Zhang, K.Z.; Li, J.W.; Xu, J.S.; Shen, Z.K.; Lin, Y.S.; Zhao, C.; Lu, X.; Rui, Y.F.; Gao, W. RBP4 promotes denervation-induced muscle atrophy through STRA6-dependent pathway. J. Cachexia Sarcopenia Muscle 2024, 15, 1601–1615. https://doi.org/10.1002/jcsm.13518.
- 116.Chang, C.L.; Li, Y.R.; Wang, Z.Y.; Li, M.L.; Jia, K.Y.; Sun, H.X.; Wang, Q.; Zhao, C.; Lu, X.; Gao, W. Serum Retinol Binding Protein 4 as a Potential Biomarker for Sarcopenia in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 34–41. https://doi.org/10.1093/gerona/glac151.
- 117.Yang, S.J.; Hwang, S.Y.; Choi, H.Y.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; Choi, D.S.; Choi, K.M. Serum selenoprotein P levels in patients with type 2 diabetes and prediabetes: Implications for insulin resistance, inflammation, and atherosclerosis. J. Clin. Endocrinol. Metab. 2011, 96, E1325–E1329. https://doi.org/10.1210/jc.2011-0620.
- 118.Misu, H.; Takayama, H.; Saito, Y.; Mita, Y.; Kikuchi, A.; Ishii, K.A.; Chikamoto, K.; Kanamori, T.; Tajima, N.; Lan, F.; et al. Deficiency of the hepatokine selenoprotein P increases responsiveness to exercise in mice through upregulation of reactive oxygen species and AMP-activated protein kinase in muscle. Nat. Med. 2017, 23, 508–516. https://doi.org/10.1038/nm.4295.
- 119.Wang, Y.; Zhao, Z.J.; Kang, X.R.; Bian, T.; Shen, Z.M.; Jiang, Y.; Sun, B.; Hu, H.B.; Chen, Y.S. lncRNA DLEU2 acts as a miR-181a sponge to regulate SEPP1 and inhibit skeletal muscle differentiation and regeneration. Aging 2020, 12, 24033–24056. https://doi.org/10.18632/aging.104095.
- 120.Abuduwaili, H.; Kamoshita, K.; Ishii, K.A.; Takahashi, K.; Abuduyimiti, T.; Qifang, L.; Isobe, Y.; Goto, H.; Nakano, Y.; Takeshita, Y.; et al. Selenoprotein P deficiency protects against immobilization-induced muscle atrophy by suppressing atrophy-related E3 ubiquitin ligases. Am. J. Physiol. Endocrinol. Metab. 2023, 324, E542–E552. https://doi.org/10.1152/ajpendo.00270.2022.
- 121.Seo, J.A.; Kang, M.C.; Yang, W.M.; Hwang, W.M.; Kim, S.S.; Hong, S.H.; Heo, J.I.; Vijyakumar, A.; Pereira de Moura, L.; Uner, A.; et al. Apolipoprotein J is a hepatokine regulating muscle glucose metabolism and insulin sensitivity. Nat. Commun. 2020, 11, 2024. https://doi.org/10.1038/s41467-020-15963-w.
- 122.Schiaffino, S.; Mammucari, C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: Insights from genetic models. Skelet. Muscle 2011, 1, 4. https://doi.org/10.1186/2044-5040-1-4.
- 123.Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970. https://doi.org/10.3390/cells9091970.
- 124.Jopling, C. Liver-specific microRNA-122: Biogenesis and function. RNA Biol. 2012, 9, 137–142. https://doi.org/10.4161/rna.18827.
- 125.Goncalves, B.S.; Meadows, A.; Pereira, D.G.; Puri, R.; Pillai, S.S. Insight into the Inter-Organ Crosstalk and Prognostic Role of Liver-Derived MicroRNAs in Metabolic Disease Progression. Biomedicines 2023, 11, 1597. https://doi.org/10.3390/biomedicines11061597.
- 126.Yan, W.; Cao, M.; Ruan, X.; Jiang, L.; Lee, S.; Lemanek, A.; Ghassemian, M.; Pizzo, D.P.; Wan, Y.; Qiao, Y.; et al. Cancer-cell-secreted miR-122 suppresses O-GlcNAcylation to promote skeletal muscle proteolysis. Nat. Cell Biol. 2022, 24, 793–804. https://doi.org/10.1038/s41556-022-00893-0.
- 127.Lan, X.Q.; Deng, C.J.; Wang, Q.Q.; Zhao, L.M.; Jiao, B.W.; Xiang, Y. The role of TGF-β signaling in muscle atrophy, sarcopenia and cancer cachexia. Gen. Comp. Endocrinol. 2024, 353, 114513. https://doi.org/10.1016/j.ygcen.2024.114513.
- 128.Borja-Gonzalez, M.; Casas-Martinez, J.C.; McDonagh, B.; Goljanek-Whysall, K. Inflamma-miR-21 Negatively Regulates Myogenesis during Ageing. Antioxidants 2020, 9, 345. https://doi.org/10.3390/antiox9040345.
- 129.Li, J.; Chan, M.C.; Yu, Y.; Bei, Y.; Chen, P.; Zhou, Q.; Cheng, L.; Chen, L.; Ziegler, O.; Rowe, G.C.; et al. miR-29b contributes to multiple types of muscle atrophy. Nat. Commun. 2017, 8, 15201. https://doi.org/10.1038/ncomms15201.
- 130.Krauss, T.; Heisz, S.; Honecker, J.; Prokopchuk, O.; Martignoni, M.; Janssen, K.P.; Claussnitzer, M.; Hauner, H.; Seeliger, C. Specific miRNAs are associated with human cancer cachexia in an organ-specific manner. J. Cachexia Sarcopenia Muscle 2023, 14, 1381–1394. https://doi.org/10.1002/jcsm.13224.
- 131.Fulzele, S.; Mendhe, B.; Khayrullin, A.; Johnson, M.; Kaiser, H.; Liu, Y.; Isales, C.M.; Hamrick, M.W. Muscle-derived miR-34a increases with age in circulating extracellular vesicles and induces senescence of bone marrow stem cells. Aging 2019, 11, 1791–1803. https://doi.org/10.18632/aging.101874.
- 132.Zhao, M.J.; Xie, J.; Shu, W.J.; Wang, H.Y.; Bi, J.; Jiang, W.; Du, H.N. MiR-15b and miR-322 inhibit SETD3 expression to repress muscle cell differentiation. Cell Death Dis. 2019, 10, 183. https://doi.org/10.1038/s41419-019-1432-5.
- 133.Kumar, A.; Davuluri, G.; Silva, R.N.E.; Engelen, M.; Ten Have, G.A.M.; Prayson, R.; Deutz, N.E.P.; Dasarathy, S. Ammonia lowering reverses sarcopenia of cirrhosis by restoring skeletal muscle proteostasis. Hepatology 2017, 65, 2045–2058. https://doi.org/10.1002/hep.29107.
- 134.Xie, Z.; Zhang, D.; Chung, D.; Tang, Z.; Huang, H.; Dai, L.; Qi, S.; Li, J.; Colak, G.; Chen, Y.; et al. Metabolic Regulation of Gene Expression by Histone Lysine β-Hydroxybutyrylation. Mol. Cell 2016, 62, 194–206. https://doi.org/10.1016/j.molcel.2016.03.036.
- 135.Zhu, Z.; Han, Z.; Halabelian, L.; Yang, X.; Ding, J.; Zhang, N.; Ngo, L.; Song, J.; Zeng, H.; He, M.; et al. Identification of lysine isobutyrylation as a new histone modification mark. Nucleic Acids Res. 2021, 49, 177–189. https://doi.org/10.1093/nar/gkaa1176.
- 136.Huang, H.; Tang, S.; Ji, M.; Tang, Z.; Shimada, M.; Liu, X.; Qi, S.; Locasale, J.W.; Roeder, R.G.; Zhao, Y.; et al. p300-Mediated Lysine 2-Hydroxyisobutyrylation Regulates Glycolysis. Mol. Cell 2018, 70, 663–678.e666. https://doi.org/10.1016/j.molcel.2018.04.011.
- 137.Mailloux, R.J.; Willmore, W.G. S-glutathionylation reactions in mitochondrial function and disease. Front. Cell Dev. Biol. 2014, 2, 68. https://doi.org/10.3389/fcell.2014.00068.
- 138.Xiao, W.; Huang, T.E.; Zhou, J.; Wang, B.; Wang, X.; Zeng, W.; Wang, Q.; Lan, X.; Xiang, Y. Inhibition of MAT2A Impairs Skeletal Muscle Repair Function. Biomolecules 2024, 14, 1098. https://doi.org/10.3390/biom14091098.
- 139.Zhang, D.; Gao, J.; Zhu, Z.; Mao, Q.; Xu, Z.; Singh, P.K.; Rimayi, C.C.; Moreno-Yruela, C.; Xu, S.; Li, G.; et al. Lysine L-lactylation is the dominant lactylation isomer induced by glycolysis. Nat. Chem. Biol. 2025, 21, 91–99. https://doi.org/10.1038/s41589-024-01680-8.
- 140.Felig, P. The glucose-alanine cycle. Metabolism 1973, 22, 179–207. https://doi.org/10.1016/0026-0495(73)90269-2.
- 141.Egami, R.; Kokaji, T.; Hatano, A.; Yugi, K.; Eto, M.; Morita, K.; Ohno, S.; Fujii, M.; Hironaka, K.I.; Uematsu, S.; et al. Trans-omic analysis reveals obesity-associated dysregulation of inter-organ metabolic cycles between the liver and skeletal muscle. iScience 2021, 24, 102217. https://doi.org/10.1016/j.isci.2021.102217.
- 142.Roslund, K.J.; Ramsey, J.J.; Rutkowsky, J.M.; Zhou, Z.; Slupsky, C.M. Two-month ketogenic diet alters systemic and brain metabolism in middle-aged female mice. Geroscience 2025, 47, 935–952. https://doi.org/10.1007/s11357-024-01314-w.
- 143.Hallan, S.I.; Øvrehus, M.A.; Darshi, M.; Montemayor, D.; Langlo, K.A.; Bruheim, P.; Sharma, K. Metabolic Differences in Diabetic Kidney Disease Patients with Normoalbuminuria versus Moderately Increased Albuminuria. Kidney360 2023, 4, 1407–1418. https://doi.org/10.34067/kid.0000000000000248.
- 144.Petersen, K.F.; Dufour, S.; Cline, G.W.; Shulman, G.I. Regulation of hepatic mitochondrial oxidation by glucose-alanine cycling during starvation in humans. J. Clin. Investig. 2019, 129, 4671–4675. https://doi.org/10.1172/jci129913.
- 145.Perry, R.J.; Wang, Y.; Cline, G.W.; Rabin-Court, A.; Song, J.D.; Dufour, S.; Zhang, X.M.; Petersen, K.F.; Shulman, G.I. Leptin Mediates a Glucose-Fatty Acid Cycle to Maintain Glucose Homeostasis in Starvation. Cell 2018, 172, 234–248.e217. https://doi.org/10.1016/j.cell.2017.12.001.
- 146.Krssak, M.; Petersen, K.F.; Bergeron, R.; Price, T.; Laurent, D.; Rothman, D.L.; Roden, M.; Shulman, G.I. Intramuscular glycogen and intramyocellular lipid utilization during prolonged exercise and recovery in man: A 13C and 1H nuclear magnetic resonance spectroscopy study. J. Clin. Endocrinol. Metab. 2000, 85, 748–754. https://doi.org/10.1210/jcem.85.2.6354.
- 147.Karpatkin, S.; Helmreich, E.; Cori, C.F. REGULATION OF GLYCOLYSIS IN MUSCLE. II. EFFECT OF STIMULATION AND EPINEPHRINE IN ISOLATED FROG SARTORIUS MUSCLE. J. Biol. Chem. 1964, 239, 3139–3145.
- 148.Sharma, A.; Oonthonpan, L.; Sheldon, R.D.; Rauckhorst, A.J.; Zhu, Z.; Tompkins, S.C.; Cho, K.; Grzesik, W.J.; Gray, L.R.; Scerbo, D.A.; et al. Impaired skeletal muscle mitochondrial pyruvate uptake rewires glucose metabolism to drive whole-body leanness. Elife 2019, 8. https://doi.org/10.7554/eLife.45873.
- 149.Visavadiya, N.P.; Rossiter, H.B.; Khamoui, A.V. Distinct glycolytic pathway regulation in liver, tumour and skeletal muscle of mice with cancer cachexia. Cell Biochem. Funct. 2021, 39, 802–812. https://doi.org/10.1002/cbf.3652.
- 150.Kulik, U.; Moesta, C.; Spanel, R.; Borlak, J. Dysfunctional Cori and Krebs cycle and inhibition of lactate transporters constitute a mechanism of primary nonfunction of fatty liver allografts. Transl. Res. 2024, 264, 33–65. https://doi.org/10.1016/j.trsl.2023.09.006.
- 151.Yan, J.; Xie, J.; Xu, S.; Guo, Y.; Ji, K.; Li, C.; Gao, H.; Zhao, L. Fibroblast growth factor 21 protects the liver from apoptosis in a type 1 diabetes mouse model via regulating L-lactate homeostasis. Biomed. Pharmacother. 2023, 168, 115737. https://doi.org/10.1016/j.biopha.2023.115737.
- 152.Murray, J.; Ehsani, A.; Najjar, L.; Zhang, G.; Itakura, K. Muscle-specific deletion of Arid5b causes metabolic changes in skeletal muscle that affect adipose tissue and liver. Front. Endocrinol. 2022, 13, 1083311. https://doi.org/10.3389/fendo.2022.1083311.
- 153.Dai, W.; Wu, G.; Liu, K.; Chen, Q.; Tao, J.; Liu, H.; Shen, M. Lactate promotes myogenesis via activating H3K9 lactylation-dependent up-regulation of Neu2 expression. J. Cachexia Sarcopenia Muscle 2023, 14, 2851–2865. https://doi.org/10.1002/jcsm.13363.
- 154.Monirujjaman, M.; Ferdouse, A. Metabolic and Physiological Roles of Branched-Chain Amino Acids. Adv. Mol. Biol. 2014, 2014, 364976. https://doi.org/10.1155/2014/364976.
- 155.Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. https://doi.org/10.1038/nrendo.2014.171.
- 156.Chan, Y.C.; Suzuki, M.; Yamamoto, S. A comparison of anthropometry, biochemical variables and plasma amino acids among centenarians, elderly and young subjects. J. Am. Coll. Nutr. 1999, 18, 358–365. https://doi.org/10.1080/07315724.1999.10718876.
- 157.Liu, H.; Zhang, Q.; Hao, Q.; Li, Q.; Yang, L.; Yang, X.; Wang, K.; Teng, J.; Gong, Z.; Jia, Y. Associations between sarcopenia and circulating branched-chain amino acids: A cross-sectional study over 100,000 participants. BMC Geriatr. 2024, 24, 541. https://doi.org/10.1186/s12877-024-05144-5.
- 158.Geriatrics Branch of the Chinese Medical Association. Guideline for diagnosis and treatment of sarcopenia in China (2024 edition). Zhonghua Yi Xue Za Zhi 2025, 105, 181–203. https://doi.org/10.3760/cma.j.cn112137-20240724-01701.
- 159.Shimomura, Y.; Yamamoto, Y.; Bajotto, G.; Sato, J.; Murakami, T.; Shimomura, N.; Kobayashi, H.; Mawatari, K. Nutraceutical effects of branched-chain amino acids on skeletal muscle. J. Nutr. 2006, 136, 529s–532s. https://doi.org/10.1093/jn/136.2.529S.
- 160.Kim, J.H.; Lee, C.; Lee, M.; Wang, H.; Kim, K.; Park, S.J.; Yoon, I.; Jang, J.; Zhao, H.; Kim, H.K.; et al. Control of leucine-dependent mTORC1 pathway through chemical intervention of leucyl-tRNA synthetase and RagD interaction. Nat. Commun. 2017, 8, 732. https://doi.org/10.1038/s41467-017-00785-0.
- 161.Wolfson, R.L.; Chantranupong, L.; Saxton, R.A.; Shen, K.; Scaria, S.M.; Cantor, J.R.; Sabatini, D.M. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 2016, 351, 43–48. https://doi.org/10.1126/science.aab2674.
- 162.Chen, J.; Ou, Y.; Luo, R.; Wang, J.; Wang, D.; Guan, J.; Li, Y.; Xia, P.; Chen, P.R.; Liu, Y. SAR1B senses leucine levels to regulate mTORC1 signalling. Nature 2021, 596, 281–284. https://doi.org/10.1038/s41586-021-03768-w.
- 163.Holeček, M. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J. Cachexia Sarcopenia Muscle 2017, 8, 529–541. https://doi.org/10.1002/jcsm.12208.
- 164.Stefan, N.; Schick, F.; Birkenfeld, A.L.; Häring, H.U.; White, M.F. The role of hepatokines in NAFLD. Cell Metab. 2023, 35, 236–252. https://doi.org/10.1016/j.cmet.2023.01.006.
- 165.Severinsen, M.C.K.; Pedersen, B.K. Muscle-Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. https://doi.org/10.1210/endrev/bnaa016.
- 166.Fisher, F.M.; Kleiner, S.; Douris, N.; Fox, E.C.; Mepani, R.J.; Verdeguer, F.; Wu, J.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E.; et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes. Dev. 2012, 26, 271–281. https://doi.org/10.1101/gad.177857.111.
- 167.Hara, H.; Uchida, S.; Yoshimura, H.; Aoki, M.; Toyoda, Y.; Sakai, Y.; Morimoto, S.; Fukamachi, H.; Shiokawa, K.; Hanada, K. Isolation and characterization of a novel liver-specific gene, hepassocin, upregulated during liver regeneration. Biochim. Biophys. Acta 2000, 1492, 31–44. https://doi.org/10.1016/s0167-4781(00)00056-7.
- 168.Hara, H.; Yoshimura, H.; Uchida, S.; Toyoda, Y.; Aoki, M.; Sakai, Y.; Morimoto, S.; Shiokawa, K. Molecular cloning and functional expression analysis of a cDNA for human hepassocin, a liver-specific protein with hepatocyte mitogenic activity. Biochim. Biophys. Acta 2001, 1520, 45–53. https://doi.org/10.1016/s0167-4781(01)00249-4.
- 169.Yu, H.T.; Yu, M.; Li, C.Y.; Zhan, Y.Q.; Xu, W.X.; Li, Y.H.; Li, W.; Wang, Z.D.; Ge, C.H.; Yang, X.M. Specific expression and regulation of hepassocin in the liver and down-regulation of the correlation of HNF1alpha with decreased levels of hepassocin in human hepatocellular carcinoma. J. Biol. Chem. 2009, 284, 13335–13347. https://doi.org/10.1074/jbc.M806393200.
- 170.Gao, M.; Zhan, Y.Q.; Yu, M.; Ge, C.H.; Li, C.Y.; Zhang, J.H.; Wang, X.H.; Ge, Z.Q.; Yang, X.M. Hepassocin activates the EGFR/ERK cascade and induces proliferation of L02 cells through the Src-dependent pathway. Cell Signal 2014, 26, 2161–2166. https://doi.org/10.1016/j.cellsig.2014.04.013.
- 171.Yang, Y.; Chen, H.; Wan, Y.; Dong, D.; Wang, X.; Yao, S.; Wang, P.; Xiang, S.; Yang, X.; Yu, M. Protective Role of Hepassocin against Hepatic Endoplasmic Reticulum Stress in Mice. Int. J. Mol. Sci. 2022, 23, 13325. https://doi.org/10.3390/ijms232113325.
- 172.Tsai, I.T.; Hung, W.C.; Lu, Y.C.; Wu, C.C.; Lee, T.L.; Hsuan, C.F.; Yu, T.H.; Wei, C.T.; Chung, F.M.; Lee, Y.J.; et al. Circulating hepassocin level in patients with stable angina is associated with fatty liver and renal function. Int. J. Med. Sci. 2021, 18, 1–7. https://doi.org/10.7150/ijms.50646.
- 173.Ketenci Gencer, F.; Yuksel, S.; Goksever Celik, H. Do serum hepassocin levels change in women with polycystic ovary syndrome? Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 267, 137–141. https://doi.org/10.1016/j.ejogrb.2021.10.034.
- 174.Toth, L.; Czigler, A.; Hegedus, E.; Komaromy, H.; Amrein, K.; Czeiter, E.; Yabluchanskiy, A.; Koller, A.; Orsi, G.; Perlaki, G.; et al. Age-related decline in circulating IGF-1 associates with impaired neurovascular coupling responses in older adults. Geroscience 2022, 44, 2771–2783. https://doi.org/10.1007/s11357-022-00623-2.
- 175.Jiang, J.J.; Chen, S.M.; Chen, J.; Wu, L.; Ye, J.T.; Zhang, Q. Serum IGF-1 levels are associated with sarcopenia in elderly men but not in elderly women. Aging Clin. Exp. Res. 2022, 34, 2465–2471. https://doi.org/10.1007/s40520-022-02180-2.
- 176.Ascenzi, F.; Barberi, L.; Dobrowolny, G.; Villa Nova Bacurau, A.; Nicoletti, C.; Rizzuto, E.; Rosenthal, N.; Scicchitano, B.M.; Musarò, A. Effects of IGF-1 isoforms on muscle growth and sarcopenia. Aging Cell 2019, 18, e12954. https://doi.org/10.1111/acel.12954.
- 177.Doi, T.; Makizako, H.; Tsutsumimoto, K.; Hotta, R.; Nakakubo, S.; Makino, K.; Suzuki, T.; Shimada, H. Association between Insulin-Like Growth Factor-1 and Frailty among Older Adults. J. Nutr. Health Aging 2018, 22, 68–72. https://doi.org/10.1007/s12603-017-0916-1.
- 178.Yang, Q.; Graham, T.E.; Mody, N.; Preitner, F.; Peroni, O.D.; Zabolotny, J.M.; Kotani, K.; Quadro, L.; Kahn, B.B. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 2005, 436, 356–362. https://doi.org/10.1038/nature03711.
- 179.Norseen, J.; Hosooka, T.; Hammarstedt, A.; Yore, M.M.; Kant, S.; Aryal, P.; Kiernan, U.A.; Phillips, D.A.; Maruyama, H.; Kraus, B.J.; et al. Retinol-binding protein 4 inhibits insulin signaling in adipocytes by inducing proinflammatory cytokines in macrophages through a c-Jun N-terminal kinase- and toll-like receptor 4-dependent and retinol-independent mechanism. Mol. Cell Biol. 2012, 32, 2010–2019. https://doi.org/10.1128/mcb.06193-11.
- 180.Bahr, M.J.; Boeker, K.H.; Manns, M.P.; Tietge, U.J. Decreased hepatic RBP4 secretion is correlated with reduced hepatic glucose production but is not associated with insulin resistance in patients with liver cirrhosis. Clin. Endocrinol. 2009, 70, 60–65. https://doi.org/10.1111/j.1365-2265.2008.03295.x.
- 181.Meex, R.C.R.; Watt, M.J. Hepatokines: Linking nonalcoholic fatty liver disease and insulin resistance. Nat. Rev. Endocrinol. 2017, 13, 509–520. https://doi.org/10.1038/nrendo.2017.56.
- 182.Teranishi, T.; Ohara, T.; Maeda, K.; Zenibayashi, M.; Kouyama, K.; Hirota, Y.; Kawamitsu, H.; Fujii, M.; Sugimura, K.; Kasuga, M. Effects of pioglitazone and metformin on intracellular lipid content in liver and skeletal muscle of individuals with type 2 diabetes mellitus. Metabolism 2007, 56, 1418–1424. https://doi.org/10.1016/j.metabol.2007.06.005.
- 183.Haukeland, J.W.; Dahl, T.B.; Yndestad, A.; Gladhaug, I.P.; Løberg, E.M.; Haaland, T.; Konopski, Z.; Wium, C.; Aasheim, E.T.; Johansen, O.E.; et al. Fetuin A in nonalcoholic fatty liver disease: In vivo and in vitro studies. Eur. J. Endocrinol. 2012, 166, 503–510. https://doi.org/10.1530/eje-11-0864.
- 184.Eswar, S.; Rajagopalan, B.; Ete, K.; Gattem, S.N.R. Clinical and Biochemical Parameters in Relation to Serum Fetuin-A Levels in Overweight and Obese with and without Metabolic Syndrome in the North-eastern States of Indian Population. JMA J. 2024, 7, 529–535. https://doi.org/10.31662/jmaj.2024-0104.
- 185.Chang, W.T.; Wu, C.H.; Hsu, L.W.; Chen, P.W.; Yu, J.R.; Chang, C.S.; Tsai, W.C.; Liu, P.Y. Serum vitamin D, intact parathyroid hormone, and Fetuin A concentrations were associated with geriatric sarcopenia and cardiac hypertrophy. Sci. Rep. 2017, 7, 40996. https://doi.org/10.1038/srep40996.
- 186.Pan, X.; Kaminga, A.C.; Chen, J.; Luo, M.; Luo, J. Fetuin-A and Fetuin-B in Non-Alcoholic Fatty Liver Disease: A Meta-Analysis and Meta-Regression. Int. J. Environ. Res. Public. Health 2020, 17, 2735. https://doi.org/10.3390/ijerph17082735.
- 187.Zhu, K.; Wang, Y.; Shu, P.; Zhou, Q.; Zhu, J.; Zhou, W.; Du, C.; Xu, C.; Liu, X.; Tang, L. Increased serum levels of fetuin B in patients with coronary artery disease. Endocrine 2017, 58, 97–105. https://doi.org/10.1007/s12020-017-1387-1.
- 188.Nagai, H.; Hamada, T.; Uchida, T.; Yamagoe, S.; Suzuki, K. Systemic expression of a newly recognized protein, LECT2, in the human body. Pathol. Int. 1998, 48, 882–886. https://doi.org/10.1111/j.1440-1827.1998.tb03855.x.
- 189.Lan, F.; Misu, H.; Chikamoto, K.; Takayama, H.; Kikuchi, A.; Mohri, K.; Takata, N.; Hayashi, H.; Matsuzawa-Nagata, N.; Takeshita, Y.; et al. LECT2 functions as a hepatokine that links obesity to skeletal muscle insulin resistance. Diabetes 2014, 63, 1649–1664. https://doi.org/10.2337/db13-0728.
- 190.Chen, C.K.; Yang, C.Y.; Hua, K.T.; Ho, M.C.; Johansson, G.; Jeng, Y.M.; Chen, C.N.; Chen, M.W.; Lee, W.J.; Su, J.L.; et al. Leukocyte cell-derived chemotaxin 2 antagonizes MET receptor activation to suppress hepatocellular carcinoma vascular invasion by protein tyrosine phosphatase 1B recruitment. Hepatology 2014, 59, 974–985. https://doi.org/10.1002/hep.26738.
- 191.Ando, K.; Kato, H.; Kotani, T.; Ozaki, M.; Arimura, Y.; Yagi, J. Plasma leukocyte cell-derived chemotaxin 2 is associated with the severity of systemic inflammation in patients with sepsis. Microbiol. Immunol. 2012, 56, 708–718. https://doi.org/10.1111/j.1348-0421.2012.00488.x.
- 192.Murphy, C.L.; Wang, S.; Kestler, D.; Larsen, C.; Benson, D.; Weiss, D.T.; Solomon, A. Leukocyte chemotactic factor 2 (LECT2)-associated renal amyloidosis: A case series. Am. J. Kidney Dis. 2010, 56, 1100–1107. https://doi.org/10.1053/j.ajkd.2010.08.013.
- 193.Misu, H.; Takamura, T.; Takayama, H.; Hayashi, H.; Matsuzawa-Nagata, N.; Kurita, S.; Ishikura, K.; Ando, H.; Takeshita, Y.; Ota, T.; et al. A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab. 2010, 12, 483–495. https://doi.org/10.1016/j.cmet.2010.09.015.
- 194.Choi, H.Y.; Hwang, S.Y.; Lee, C.H.; Hong, H.C.; Yang, S.J.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; et al. Increased selenoprotein p levels in subjects with visceral obesity and nonalcoholic Fatty liver disease. Diabetes Metab. J. 2013, 37, 63–71. https://doi.org/10.4093/dmj.2013.37.1.63.
- 195.Argemi, J.; Kedia, K.; Gritsenko, M.A.; Clemente-Sanchez, A.; Asghar, A.; Herranz, J.M.; Liu, Z.X.; Atkinson, S.R.; Smith, R.D.; Norden-Krichmar, T.M.; et al. Integrated Transcriptomic and Proteomic Analysis Identifies Plasma Biomarkers of Hepatocellular Failure in Alcohol-Associated Hepatitis. Am. J. Pathol. 2022, 192, 1658–1669. https://doi.org/10.1016/j.ajpath.2022.08.009.
- 196.Chen, T.C.; Huang, T.H.; Tseng, W.C.; Tseng, K.W.; Hsieh, C.C.; Chen, M.Y.; Chou, T.Y.; Huang, Y.C.; Chen, H.L.; Nosaka, K. Changes in plasma C1q, apelin and adropin concentrations in older adults after descending and ascending stair walking intervention. Sci. Rep. 2021, 11, 17644. https://doi.org/10.1038/s41598-021-96631-x.
- 197.Xu, A.; Lam, M.C.; Chan, K.W.; Wang, Y.; Zhang, J.; Hoo, R.L.; Xu, J.Y.; Chen, B.; Chow, W.S.; Tso, A.W.; et al. Angiopoietin-like protein 4 decreases blood glucose and improves glucose tolerance but induces hyperlipidemia and hepatic steatosis in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 6086–6091. https://doi.org/10.1073/pnas.0408452102.
- 198.Chang, H.; Kwon, O.; Shin, M.S.; Kang, G.M.; Leem, Y.H.; Lee, C.H.; Kim, S.J.; Roh, E.; Kim, H.K.; Youn, B.S.; et al. Role of Angptl4/Fiaf in exercise-induced skeletal muscle AMPK activation. J. Appl. Physiol. 2018, 125, 715–722. https://doi.org/10.1152/japplphysiol.00984.2016.
- 199.Miller, L.L.; Bly, C.G.; Watson, M.L.; Bale, W.F. The dominant role of the liver in plasma protein synthesis; a direct study of the isolated perfused rat liver with the aid of lysine-epsilon-C14. J. Exp. Med. 1951, 94, 431–453. https://doi.org/10.1084/jem.94.5.431.
- 200.Tüfekci, K.U.; Oner, M.G.; Meuwissen, R.L.; Genç, S. The role of microRNAs in human diseases. Methods Mol. Biol. 2014, 1107, 33–50. https://doi.org/10.1007/978-1-62703-748-8_3.
- 201.Hwang, H.W.; Mendell, J.T. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br. J. Cancer 2006, 94, 776–780. https://doi.org/10.1038/sj.bjc.6603023.
- 202.Rottiers, V.; Näär, A.M. MicroRNAs in metabolism and metabolic disorders. Nat. Rev. Mol. Cell Biol. 2012, 13, 239–250. https://doi.org/10.1038/nrm3313.
- 203.Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–d162. https://doi.org/10.1093/nar/gky1141.
- 204.Chen, Y.; Verfaillie, C.M. MicroRNAs: The fine modulators of liver development and function. Liver Int. 2014, 34, 976–990. https://doi.org/10.1111/liv.12496.
- 205.Maes, O.C.; An, J.; Sarojini, H.; Wang, E. Murine microRNAs implicated in liver functions and aging process. Mech. Ageing Dev. 2008, 129, 534–541. https://doi.org/10.1016/j.mad.2008.05.004.
- 206.Szabo, G.; Bala, S. MicroRNAs in liver disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 542–552. https://doi.org/10.1038/nrgastro.2013.87.
- 207.Bernstein, E.; Kim, S.Y.; Carmell, M.A.; Murchison, E.P.; Alcorn, H.; Li, M.Z.; Mills, A.A.; Elledge, S.J.; Anderson, K.V.; Hannon, G.J. Dicer is essential for mouse development. Nat. Genet. 2003, 35, 215–217. https://doi.org/10.1038/ng1253.
- 208.Wang, Y.; Medvid, R.; Melton, C.; Jaenisch, R.; Blelloch, R. DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nat. Genet. 2007, 39, 380–385. https://doi.org/10.1038/ng1969.
- 209.Horak, M.; Novak, J.; Bienertova-Vasku, J. Muscle-specific microRNAs in skeletal muscle development. Dev. Biol. 2016, 410, 1–13. https://doi.org/10.1016/j.ydbio.2015.12.013.
- 210.Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. https://doi.org/10.1038/ncb1596.
- 211.Sheta, M.; Taha, E.A.; Lu, Y.; Eguchi, T. Extracellular Vesicles: New Classification and Tumor Immunosuppression. Biology 2023, 12, 110. https://doi.org/10.3390/biology12010110.
- 212.Johnstone, R.M.; Bianchini, A.; Teng, K. Reticulocyte maturation and exosome release: Transferrin receptor containing exosomes shows multiple plasma membrane functions. Blood 1989, 74, 1844–1851.
- 213.Robbins, P.D.; Morelli, A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. https://doi.org/10.1038/nri3622.
- 214.Aatonen, M.T.; Ohman, T.; Nyman, T.A.; Laitinen, S.; Grönholm, M.; Siljander, P.R. Isolation and characterization of platelet-derived extracellular vesicles. J. Extracell. Vesicles 2014, 3. https://doi.org/10.3402/jev.v3.24692.
- 215.Fang, X.; Zhang, Y.; Zhang, Y.; Guan, H.; Huang, X.; Miao, R.; Yin, R.; Tian, J. Endothelial extracellular vesicles: Their possible function and clinical significance in diabetic vascular complications. J. Transl. Med. 2024, 22, 944. https://doi.org/10.1186/s12967-024-05760-0.
- 216.Pitzer, C.R.; Paez, H.G.; Alway, S.E. The Contribution of Tumor Derived Exosomes to Cancer Cachexia. Cells 2023, 12, 292. https://doi.org/10.3390/cells12020292.
- 217.Zhou, L.; Zhang, T.; Shao, W.; Lu, R.; Wang, L.; Liu, H.; Jiang, B.; Li, S.; Zhuo, H.; Wang, S.; et al. Amiloride ameliorates muscle wasting in cancer cachexia through inhibiting tumor-derived exosome release. Skelet. Muscle 2021, 11, 17. https://doi.org/10.1186/s13395-021-00274-5.
- 218.Povero, D.; Eguchi, A.; Niesman, I.R.; Andronikou, N.; de Mollerat du Jeu, X.; Mulya, A.; Berk, M.; Lazic, M.; Thapaliya, S.; Parola, M.; et al. Lipid-induced toxicity stimulates hepatocytes to release angiogenic microparticles that require Vanin-1 for uptake by endothelial cells. Sci. Signal 2013, 6, ra88. https://doi.org/10.1126/scisignal.2004512.
- 219.Hou, J.; Lin, L.; Zhou, W.; Wang, Z.; Ding, G.; Dong, Q.; Qin, L.; Wu, X.; Zheng, Y.; Yang, Y.; et al. Identification of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinoma. Cancer Cell 2011, 19, 232–243. https://doi.org/10.1016/j.ccr.2011.01.001.
- 220.Gamazon, E.R.; Innocenti, F.; Wei, R.; Wang, L.; Zhang, M.; Mirkov, S.; Ramírez, J.; Huang, R.S.; Cox, N.J.; Ratain, M.J.; et al. A genome-wide integrative study of microRNAs in human liver. BMC Genomics 2013, 14, 395. https://doi.org/10.1186/1471-2164-14-395.
- 221.Roderburg, C.; Urban, G.W.; Bettermann, K.; Vucur, M.; Zimmermann, H.; Schmidt, S.; Janssen, J.; Koppe, C.; Knolle, P.; Castoldi, M.; et al. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology 2011, 53, 209–218. https://doi.org/10.1002/hep.23922.
- 222.Sun, Y.; Wang, H.; Li, Y.; Liu, S.; Chen, J.; Ying, H. miR-24 and miR-122 Negatively Regulate the Transforming Growth Factor-β/Smad Signaling Pathway in Skeletal Muscle Fibrosis. Mol. Ther. Nucleic Acids 2018, 11, 528–537. https://doi.org/10.1016/j.omtn.2018.04.005.
- 223.Song, X.; Liu, F.; Chen, M.; Zhu, M.; Zheng, H.; Wang, W.; Chen, D.; Li, M.; Chen, S. MiR-21 regulates skeletal muscle atrophy and fibrosis by targeting TGF-beta/SMAD7-SMAD2/3 signaling pathway. Heliyon 2024, 10, e33062. https://doi.org/10.1016/j.heliyon.2024.e33062.
- 224.Burgess, K.S.; Philips, S.; Benson, E.A.; Desta, Z.; Gaedigk, A.; Gaedigk, R.; Segar, M.W.; Liu, Y.; Skaar, T.C. Age-Related Changes in MicroRNA Expression and Pharmacogenes in Human Liver. Clin. Pharmacol. Ther. 2015, 98, 205–215. https://doi.org/10.1002/cpt.145.
- 225.Baker, S.A.; Rutter, J. Metabolites as signalling molecules. Nat. Rev. Mol. Cell Biol. 2023, 24, 355–374. https://doi.org/10.1038/s41580-022-00572-w.
- 226.Costa Dos Santos, G., Jr.; Renovato-Martins, M.; de Brito, N.M. The remodel of the “central dogma”: A metabolomics interaction perspective. Metabolomics 2021, 17, 48. https://doi.org/10.1007/s11306-021-01800-8.
- 227.Zhong, Q.; Xiao, X.; Qiu, Y.; Xu, Z.; Chen, C.; Chong, B.; Zhao, X.; Hai, S.; Li, S.; An, Z.; et al. Protein posttranslational modifications in health and diseases: Functions, regulatory mechanisms, and therapeutic implications. MedComm 2023, 4, e261. https://doi.org/10.1002/mco2.261.
- 228.He, X.; Wei, Y.; Wu, J.; Wang, Q.; Bergholz, J.S.; Gu, H.; Zou, J.; Lin, S.; Wang, W.; Xie, S.; et al. Lysine vitcylation is a novel vitamin C-derived protein modification that enhances STAT1-mediated immune response. bioRxiv 2023. https://doi.org/10.1101/2023.06.27.546774.
- 229.Zhong, Q.; Zheng, K.; Li, W.; An, K.; Liu, Y.; Xiao, X.; Hai, S.; Dong, B.; Li, S.; An, Z.; et al. Post-translational regulation of muscle growth, muscle aging and sarcopenia. J. Cachexia Sarcopenia Muscle 2023, 14, 1212–1227. https://doi.org/10.1002/jcsm.13241.
- 230.Markofski, M.M.; Dickinson, J.M.; Drummond, M.J.; Fry, C.S.; Fujita, S.; Gundermann, D.M.; Glynn, E.L.; Jennings, K.; Paddon-Jones, D.; Reidy, P.T.; et al. Effect of age on basal muscle protein synthesis and mTORC1 signaling in a large cohort of young and older men and women. Exp. Gerontol. 2015, 65, 1–7. https://doi.org/10.1016/j.exger.2015.02.015.
- 231.Doran, P.; Gannon, J.; O’Connell, K.; Ohlendieck, K. Aging skeletal muscle shows a drastic increase in the small heat shock proteins alphaB-crystallin/HspB5 and cvHsp/HspB7. Eur. J. Cell Biol. 2007, 86, 629–640. https://doi.org/10.1016/j.ejcb.2007.07.003.
- 232.Ling, B.M.; Bharathy, N.; Chung, T.K.; Kok, W.K.; Li, S.; Tan, Y.H.; Rao, V.K.; Gopinadhan, S.; Sartorelli, V.; Walsh, M.J.; et al. Lysine methyltransferase G9a methylates the transcription factor MyoD and regulates skeletal muscle differentiation. Proc. Natl. Acad. Sci. USA 2012, 109, 841–846. https://doi.org/10.1073/pnas.1111628109.
- 233.Song, R.; Peng, W.; Zhang, Y.; Lv, F.; Wu, H.K.; Guo, J.; Cao, Y.; Pi, Y.; Zhang, X.; Jin, L.; et al. Central role of E3 ubiquitin ligase MG53 in insulin resistance and metabolic disorders. Nature 2013, 494, 375–379. https://doi.org/10.1038/nature11834.
- 234.Bandyopadhyay, G.K.; Yu, J.G.; Ofrecio, J.; Olefsky, J.M. Increased malonyl-CoA levels in muscle from obese and type 2 diabetic subjects lead to decreased fatty acid oxidation and increased lipogenesis; thiazolidinedione treatment reverses these defects. Diabetes 2006, 55, 2277–2285. https://doi.org/10.2337/db06-0062.
- 235.Tang, H.; Cui, M.; Han, M. Fatty acids impact sarcomere integrity through myristoylation and ER homeostasis. Cell Rep. 2021, 36, 109539. https://doi.org/10.1016/j.celrep.2021.109539.
- 236.Jaisson, S.; Pietrement, C.; Gillery, P. Protein Carbamylation: Chemistry, Pathophysiological Involvement, and Biomarkers. Adv. Clin. Chem. 2018, 84, 1–38. https://doi.org/10.1016/bs.acc.2017.12.001.
- 237.Badar, A.; Arif, Z.; Alam, K. Role of Carbamylated Biomolecules in Human Diseases. IUBMB Life 2018, 70, 267–275. https://doi.org/10.1002/iub.1732.
- 238.Wang, Z.; Nicholls, S.J.; Rodriguez, E.R.; Kummu, O.; Hörkkö, S.; Barnard, J.; Reynolds, W.F.; Topol, E.J.; DiDonato, J.A.; Hazen, S.L. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat. Med. 2007, 13, 1176–1184. https://doi.org/10.1038/nm1637.
- 239.Gorisse, L.; Pietrement, C.; Vuiblet, V.; Schmelzer, C.E.; Köhler, M.; Duca, L.; Debelle, L.; Fornès, P.; Jaisson, S.; Gillery, P. Protein carbamylation is a hallmark of aging. Proc. Natl. Acad. Sci. USA 2016, 113, 1191–1196. https://doi.org/10.1073/pnas.1517096113.
- 240.Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. https://doi.org/10.1093/jn/134.3.489.
- 241.Stramentinoli, G.; Gualano, M.; Catto, E.; Algeri, S. Tissue levels of S-adenosylmethionine in aging rats. J. Gerontol. 1977, 32, 392–394. https://doi.org/10.1093/geronj/32.4.392.
- 242.Hayashi, Y.; Kashio, S.; Murotomi, K.; Hino, S.; Kang, W.; Miyado, K.; Nakao, M.; Miura, M.; Kobayashi, S.; Namihira, M. Biosynthesis of S-adenosyl-methionine enhances aging-related defects in Drosophila oogenesis. Sci. Rep. 2022, 12, 5593. https://doi.org/10.1038/s41598-022-09424-1.
- 243.Xie, T.; Leung, P.S. Fibroblast growth factor 21: A regulator of metabolic disease and health span. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E292–e302. https://doi.org/10.1152/ajpendo.00101.2017.
- 244.Villarroya, J.; Gallego-Escuredo, J.M.; Delgado-Anglés, A.; Cairó, M.; Moure, R.; Gracia Mateo, M.; Domingo, J.C.; Domingo, P.; Giralt, M.; Villarroya, F. Aging is associated with increased FGF21 levels but unaltered FGF21 responsiveness in adipose tissue. Aging Cell 2018, 17, e12822. https://doi.org/10.1111/acel.12822.
- 245.Qiu, X.; Wu, W.; Zhang, S.; Huang, C.; Lin, D. 3-Hydroxybutyrate Promotes Myoblast Proliferation and Differentiation through Energy Metabolism and GPR109a-Mediated Ca2+-NFAT Signaling Pathways. J. Proteome Res. 2025, 24, 2063–2080. https://doi.org/10.1021/acs.jproteome.4c01150.
- 246.Chen, J.; Li, Z.; Zhang, Y.; Zhang, X.; Zhang, S.; Liu, Z.; Yuan, H.; Pang, X.; Liu, Y.; Tao, W.; et al. Mechanism of reduced muscle atrophy via ketone body (D)-3-hydroxybutyrate. Cell Biosci. 2022, 12, 94. https://doi.org/10.1186/s13578-022-00826-2.
- 247.Qiu, X.; Zou, Z.; Lin, T.; Guo, C.; Lin, D. Engineered Lactobacillus rhamnosus Producing 3-Hydroxybutyrate: A Dual-Action Therapeutic Strategy for Colon Cancer Cachexia. Biotechnol. Bioeng. 2025. https://doi.org/10.1002/bit.28972.
- 248.Xiang, Y.; Wang, Q.-Q.; Lan, X.-Q.; Zhang, H.-J.; Wei, D.-X. Function and treatment strategies of β-hydroxybutyrate in aging. Smart Mater. Med. 2023, 4, 160–172. https://doi.org/10.1016/j.smaim.2022.09.003.
- 249.Perakakis, N.; Stefanakis, K.; Feigh, M.; Veidal, S.S.; Mantzoros, C.S. Elafibranor and liraglutide improve differentially liver health and metabolism in a mouse model of non-alcoholic steatohepatitis. Liver Int. 2021, 41, 1853–1866. https://doi.org/10.1111/liv.14888.
- 250.Hong, Y.; Lee, J.H.; Jeong, K.W.; Choi, C.S.; Jun, H.S. Amelioration of muscle wasting by glucagon-like peptide-1 receptor agonist in muscle atrophy. J. Cachexia Sarcopenia Muscle 2019, 10, 903–918. https://doi.org/10.1002/jcsm.12434.
- 251.Song, Y.; Ni, W.; Zheng, M.; Sheng, H.; Wang, J.; Xie, S.; Yang, Y.; Chi, X.; Chen, J.; He, F.; et al. Vitamin E (300 mg) in the treatment of MASH: A multi-center, randomized, double-blind, placebo-controlled study. Cell Rep. Med. 2025, 6, 101939. https://doi.org/10.1016/j.xcrm.2025.101939.
- 252.Sacheck, J.M.; Blumberg, J.B. Role of vitamin E and oxidative stress in exercise. Nutrition 2001, 17, 809–814. https://doi.org/10.1016/s0899-9007(01)00639-6.
- 253.Shen, Y.; Cheng, L.; Xu, M.; Wang, W.; Wan, Z.; Xiong, H.; Guo, W.; Cai, M.; Xu, F. SGLT2 inhibitor empagliflozin downregulates miRNA-34a-5p and targets GREM2 to inactivate hepatic stellate cells and ameliorate non-alcoholic fatty liver disease-associated fibrosis. Metabolism 2023, 146, 155657. https://doi.org/10.1016/j.metabol.2023.155657.
- 254.Huang, Q.; Chen, J.; Liao, S.; Long, J.; Fang, R.; He, Y.; Chen, P.; Liu, D. The SGLT2 inhibitor empagliflozin inhibits skeletal muscle fibrosis in naturally aging male mice through the AMPKα/MMP9/TGF-β1/Smad pathway. Biogerontology 2024, 25, 567–581. https://doi.org/10.1007/s10522-024-10093-y.
- 255.Kaspy, M.S.; Hannaian, S.J.; Bell, Z.W.; Churchward-Venne, T.A. The effects of branched-chain amino acids on muscle protein synthesis, muscle protein breakdown and associated molecular signalling responses in humans: An update. Nutr. Res. Rev. 2024, 37, 273–286. https://doi.org/10.1017/s0954422423000197.
- 256.van Dijk, A.M.; Bruins Slot, A.S.; Portincasa, P.; Siegerink, S.N.; Chargi, N.; Verstraete, C.J.R.; de Bruijne, J.; Vleggaar, F.P.; van Erpecum, K.J. Systematic review with meta-analysis: Branched-chain amino acid supplementation in liver disease. Eur. J. Clin. Investig. 2023, 53, e13909. https://doi.org/10.1111/eci.13909.
- 257.Ali, F.F.; Rifaai, R.A. Preventive effect of omega-3 fatty acids in a rat model of stress-induced liver injury. J. Cell Physiol. 2019, 234, 11960–11968. https://doi.org/10.1002/jcp.27848.
- 258.Kim, H.J.; So, B.; Choi, M.; Kang, D.; Song, W. Resistance exercise training increases the expression of irisin concomitant with improvement of muscle function in aging mice and humans. Exp. Gerontol. 2015, 70, 11–17. https://doi.org/10.1016/j.exger.2015.07.006.
- 259.So, W.Y.; Leung, P.S. Irisin ameliorates hepatic glucose/lipid metabolism and enhances cell survival in insulin-resistant human HepG2 cells through adenosine monophosphate-activated protein kinase signaling. Int. J. Biochem. Cell Biol. 2016, 78, 237–247. https://doi.org/10.1016/j.biocel.2016.07.022.
- 260.Pedersen, B.K.; Febbraio, M. Muscle-derived interleukin-6--a possible link between skeletal muscle, adipose tissue, liver, and brain. Brain Behav. Immun. 2005, 19, 371–376. https://doi.org/10.1016/j.bbi.2005.04.008.
How to Cite
Wang, Q.; Yao, M.; Wang, X.; Lan, X.; Fan, G.; Xiang, Y. From Liver to Muscle: Crosstalk Mechanisms and Interventions in Sarcopenia. Health and Metabolism 2025, 2 (3), 7. https://doi.org/10.53941/hm.2025.100022.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


