Selective glycoengineering of tumors in vivo is a highly promising strategy for tumor treatment. Although metabolic glycan labeling using precursors of sialic acid (Sia) is an effective method for attaching chemical probes to cell surfaces, it often lacks specificity for tumors. Herein, we report a tumor-activated sialoengineering approach utilizing Sia with C1-carboxylate caged with lysine (K). Probe decaging by carboxypeptidase in tumors enables tumor-exofacial expression of abiotic Sia tolerating differing C9-substitutions such as azide (AzSia) and m-phenoxybenzamide (PBASia). Notably, PBASia acts as a high-affinity ligand for the CD22 receptor of B cells. Treatment with PBASia-K leads to robust suppression of subcutaneous B16-F10 tumors in mice. These data show the potential of C1-caged Sia to function as a generic small-molecule platform for in vivo sialoengineering of tumor cells, allowing for the generation of cell surface-anchored C9-substituted Sia that could be harnessed to stimulate an anti-tumor response.
- Open Access
- Article
A Sialic Acid-Caged Generic Platform for Sialoengineering of Tumors with Artificial Immuno-Ligand
- Shuo Zhang 1, †,
- Huiling Dong 2, †,
- Shixiong Wen 3, 4,
- Zejing Lin 1,
- Xuanjun Wu 2, *,
- Jiahuai Han 3, 4, *,
- Shoufa Han 1, 3, *
Author Information
Received: 16 Jun 2025 | Revised: 27 Jun 2025 | Accepted: 04 Jul 2025 | Published: 30 Jul 2025
Abstract
Graphical Abstract
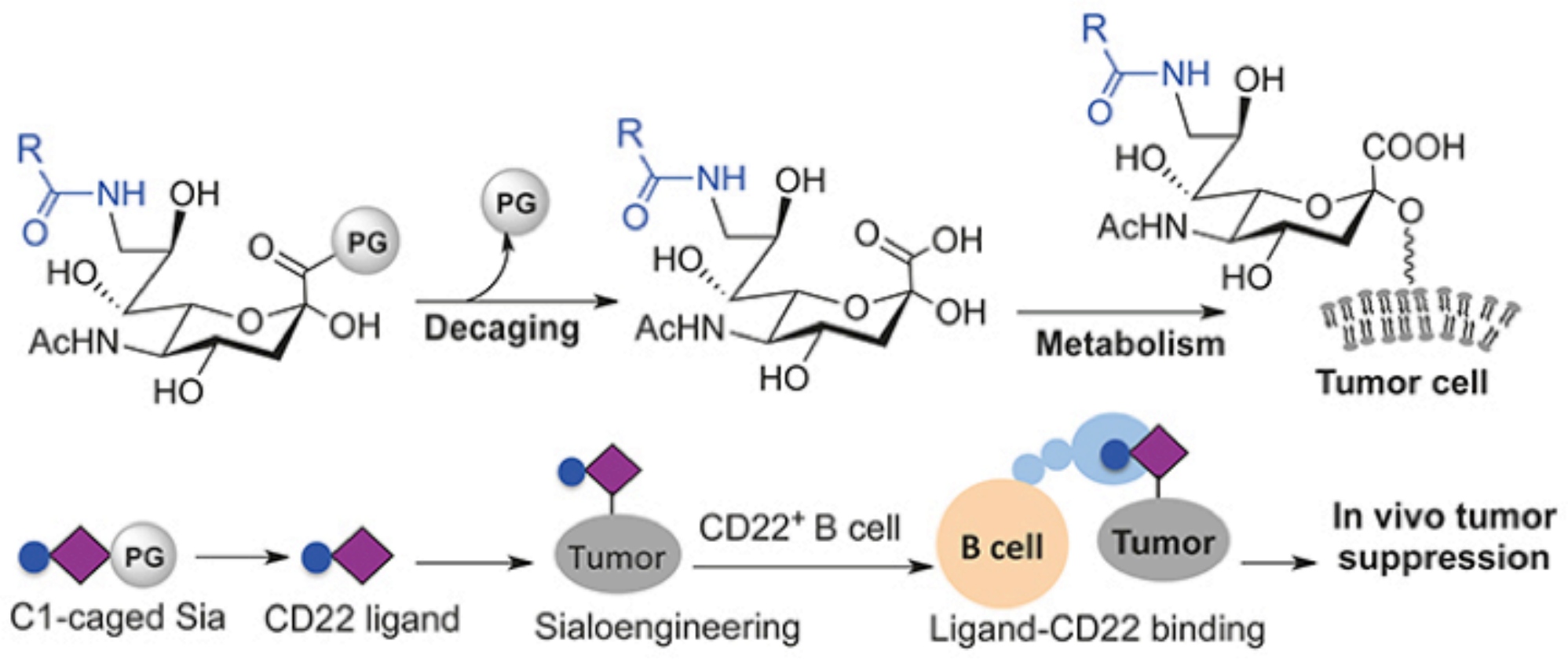
Keywords
metabolic engineering | sialylation | decaging | CD22 high-affinity ligand | tumor suppression
References
- 1.Shmeeda, H.; Mak, L.; Tzemach, D.; Astrahan, P.; Tarshish, M.; Gabizon, A. Intracellular uptake and intracavitary targeting of folate-conjugated liposomes in a mouse lymphoma model with up-regulated folate receptors. Mol. Cancer Ther. 2006, 5, 818–824.
- 2.Rouault, H.; Hakim, V. Different cell fates from cell-cell interactions: Core architectures of two-cell bistable networks. Biophys. J. 2012, 102, 417–426.
- 3.Monge, P.; Søgaard, A.B.; Andersen, D.G.; Chandrawati, R.; Zelikin, A.N.. Synthetic chemical ligands and cognate antibodies for biorthogonal drug targeting and cell engineering. Adv. Drug Deliv. Rev. 2021, 170, 281–293.
- 4.Guryanov, I.; Fiorucci, S.; Tennikova, T. Receptor-ligand interactions: Advanced biomedical applications. Mat. Sci. Eng. C 2016, 68, 890–903.
- 5.Angata, T.; Varki, A. Chemical diversity in the sialic acids and related alpha-keto acids: An evolutionary perspective. Chem. Rev. 2002, 102, 439–469.
- 6.Prescher, J.A.; Dube, D.H.; Bertozzi, C.R. Chemical remodelling of cell surfaces in living animals. Nature 2004, 430, 873–877.
- 7.Wang, H.M.; Mooney, D.J. Metabolic glycan labelling for cancer-targeted therapy. Nat. Chem. 2020, 12, 1102–1114.
- 8.Kufleitner, M.; Haiber, L.M.; Wittmann, V. Metabolic glycoengineering—exploring glycosylation with bioorthogonal chemistry. Chem. Soc. Rev. 2023, 52, 510–535.
- 9.Ai, X.; Lyu, L.; Zhang, Y.; Rong, J.; Chen, X. Remote regulation of membrane channel activity by site-specific localization of lanthanid-doped upconversion nanocrystals. Angew. Chem. Int. Ed. 2017, 56, 3031–3035.
- 10.Agatemor, C.; Buettner, M.J.; Ariss, R.; Muthiah, K.; Saeui, C.T.; Yarema, K.J. Exploiting metabolic glycoengineering to advance healthcare. Nat. Rev. Chem. 2019, 3, 605–620.
- 11.Sletten, E.M.; Bertozzi, C.R. Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality. Angew. Chem. Int. Ed. 2009, 48, 6974–6998.
- 12.Hu, M.; Han, Q.; Lyu, L.; Tong, Y.; Dong, S.; Loh, Z.H.; Xing, B. Luminescent molecules towards precise cellular event regulation. Chem. Commun. 2020, 56, 10231–10234.
- 13.Droujinine, I.A.; Meyer, A.S.; Wang, D.; Udeshi, N.D.; Hu, Y.; Rocco, D.; Perrimon, N. Proteomics of protein trafficking by in vivo tissue-specific labeling. Nat. Commun. 2021, 12, 2382.
- 14.Sampathkumar, S.G.; Li, A.V.; Jones, M.B.; Sun, Z.; Yarema, K.J. Metabolic installation of thiols into sialic acid modulates adhesion and stem cell biology. Nat. Chem. Biol. 2006, 2, 149–152. https://doi.org/10.1038/nchembio770.
- 15.Saxon, E.; Bertozzi, C.R. Cell surface engineering by a modified Staudinger reaction. Science 2000, 287, 2007–2010.
- 16.Hong, S.; Sahai-Hernandez, P.; Chapla, D.G.; Moremen, K.W.; Traver, D.; Wu, P. Direct Visualization of Live Zebrafish Glycans via Single-Step Metabolic Labeling with Fluorophore-Tagged Nucleotide Sugars. Angew. Chem. Int. Ed. 2019, 58, 14327–14333.
- 17.Xie, R.; Hong, S.; Feng, L.; Rong, J.; Chen, X. Cell-selective metabolic glycan labeling based on ligand-targeted liposomes. J. Am. Chem. Soc. 2012, 134, 9914.
- 18.Xie, R.; Dong, L.; Huang, R.; Hong, S.; Lei, R.; Chen, X. Targeted imaging and proteomic analysis of tumor-associated glycans in living animals. Angew. Chem. Int. Ed. 2014, 53, 14082–14086.
- 19.BDebets, M.F. Metabolic precision labeling enables selective probing of O-linked N-acetylgalactosamine glycosylation. Proc. Nat. Acad. Sci. USA 2020, 117, 25293–25301.
- 20.Cioce, A.; Calle, B.; Rizou, T.; Lowery, S.C.; Bridgeman, V.L.; Mahoney, K.E.; Schumann, B. Cell-specific bioorthogonal tagging of glycoproteins. Nat. Commun. 2022, 12, 6237.
- 21.Wang, H.; Sobral, M.C.; Zhang, D.K.; Cartwright, A.N.; Li, A.W.; Dellacherie, M.O.; Mooney, D.J. Metabolic labeling and targeted modulation of dendritic cells. Nat. Mater. 2020, 19, 1244–1252.
- 22.Wang, H.; Gauthier, M.; Kelly, J.R.; Xu, M.; O’Brien, W.D., Jr.; Cheng, J. Targeted ultrasound-assisted cancer-selective chemical labeling and subsequent cancer imaging using click chemistry. Angewandte Chemie International Edition. Angew. Chem. Int. Ed. 2016, 55, 5452–5456.
- 23.Wang, R.; Cai, K.; Wang, H.; Yin, C.; Cheng, J. A caged metabolic precursor for DT-diaphorase- responsive cell labeling. Chem. Commun. 2018, 54, 4878–4881.
- 24.Wang, H.; Wang, R.; Cai, K.; He, H.; Liu, Y.; Yen, J.; Cheng, J. selective in vivo metabolic cell-labeling-mediated cancer targeting. Nat. Chem. Biol. 2017, 13, 415–424.
- 25.Wang, Z.; Lau, J.W.; Liu, S.; Ren, Z.; Gong, Z.; Liu, X.; Xing, B. A Nitroreductase-Activatable Metabolic Reporter for Covalent Labeling of Pathological Hypoxic Cells in Tumorigenesis. Angew. Chem. Int. Ed. 2024, 136, e202411636.
- 26.Shim, M.K.; Yoon, H.Y.; Ryu, J.H.; Koo, H.; Lee, S.; Park, J.H.; Kim, K. Cathepsin B-specific metabolic precursor for in vivo tumor-specific fluorescence imaging. Angew. Chem. Int. Ed. 2016, 55, 14698–14703.
- 27.Rillahan, C.D.; Macauley, M.S.; Schwartz, E.; He, Y.; McBride, R.; Arlian, B.M.; Paulson, J.C. Disubstituted sialic acid ligands targeting siglecs CD33 and CD22 associated with myeloid leukaemias and B cell lymphomas. Chem. Sci. 2014, 5, 2398–2406.
- 28.Wang, X.; Luo, X.; Tian, Y.; Wu, T.; Weng, J.; Li, Z.; Huang, X. Equipping Natural Killer Cells with Cetuximab through Metabolic Glycoengineering and Bioorthogonal Reaction for Targeted Treatment of KRAS Mutant Colorectal Cancer. ACS Chem. Biol. 2012, 16, 724–730.
- 29.Wang, X.; Lang, S.; Tian, Y.; Zhang, J.; Yan, X.; Fang, Z.; Huang, X. Glycoengineering of natural killer cells with CD22 ligands for enhanced anticancer immunotherapy. ACS Cent. Sci. 2020, 6, 382–389.
- 30.Han, S.; Collins, B.E.; Bengtson, P.; Paulson, J.C. Homomultimeric complexes of CD22 in B cells revealed by protein-glycan cross-linking. Nat. Chem. Biol. 2005, 1, 93–97. https://doi.org/10.1038/nchembio713.
- 31.Lin, B.; Wu, X.; Zhao, H.; Tian, Y.; Han, J.; Liu, J.; Han, S. Redirecting immunity via covalently incorporated immunogenic sialic acid on the tumor cell surface. Chem. Sci. 2016, 7, 3737.
- 32.Olson, W.C.; Heston, W.D.; Rajasekaran, A.K. Clinical trials of cancer therapies targeting prostate-specific membrane antigen. Rev. Recent Clin. Trials 2007, 2, 182–190.
- 33.Cawley, N.X.; Wetsel, W.C.; Murthy, S.R.K.; Park, J.J.; Pacak, K.; Loh, Y.P. New roles of carboxypeptidase E in endocrine and neural function and cancer. Endocr. Rev. 2012, 33, 216–153.
- 34.DiJoseph, J.F.; Dougher, M.M.; Armellino, D.C.; Evans, D.Y.; Damle, N.K. Therapeutic potential of CD22- specific antibody-targeted chemotherapy using inotuzumab ozogamicin (CMC-544) for the treatment of acute lymphoblastic leukemia. Leukemia 2007, 21, 2240–2245.
- 35.Kreitman, R.J.; Margulies, I.; Stetler-Stevenson, M.; Wang, Q.C.; FitzGerald, D.J.; Pastan, I. Cytotoxic activity of disulfide-stabilized recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) toward fresh malignant cells from patients with B-cell leukemias. Clin. Cancer Res. 2000, 6, 1476–1487.
- 36.Kreitman, R.J.; Wilson, W.H.; Bergeron, K.; Raggio, M.; Stetler-Stevenson, M.; FitzGerald, D.J.; Pastan, I. Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. N. Engl. J. Med. 2001, 345, 241–247.
- 37.Fry, T.J.; Shah, N.N.; Orentas, R.J.; Stetler-Stevenson, M.; Yuan, C.M.; Ramakrishna, S.; Mackall, C.L. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 2018, 24, 20–28.
- 38.Ramakrishna, S.; Highfill, S.L.; Walsh, Z.; Nguyen, S.M.; Lei, H.; Shern, J.F.; Fry, T.J. Modulation of target antigen density improves CAR T-cell functionality and persistence. Clin. Cancer Res. 2019, 25, 5329–5341.
- 39.Zhang, Y.; Gallastegui, N.; Rosenblatt, J.D. Regulatory B cells in anti-tumor immunity. Int. Immunol. 2015, 27, 521–530.
- 40.Qin, Y.; Lu, F.; Lyu, K.; Chang, A.E.; Li, Q. Emerging concepts regarding pro-and anti tumor properties of B cells in tumor immunity. Front. Immunol. 2022, 13, 881427.
- 41.Sarvaria, A.; Madrigal, J.A.; Saudemont, A. B cell regulation in cancer and anti-tumor immunity. Cellular & molecular immunology. Cell. Mol. Immunol. 2017, 14, 662–674.
- 42.Wang, S.S.; Liu, W.; Ly, D.; Xu, H.; Qu, L.; Zhang, L. Tumor-infiltrating B cells: Their role and application in anti-tumor immunity in lung cancer. Cell. Mol. Immunol. 2019, 16, 6–18.
- 43.Kinoshita, Y.; Sato, S.; Takeuchi, T. Cellular sialic acid level and phenotypic expression in B16 melanoma cells: Comparison of spontaneous variations and bromodeoxyuridine and theophylline-induced changes. Cell Struct. Funct. 1989, 14, 35–43.
- 44.Wieboldt, R.; Sandholzer, M.; Carlini, E.; Lin, C.W.; Börsch, A.; Zingg, A.; Mantuano, N.R. Engagement of sialylated glycans with Siglec receptors on suppressive myeloid cells inhibits anticancer immunity via CCL2. Cell Mol. Immunol. 2024, 21, 495–509.
- 45.Kang, J.; Sun, M.; Chang, Y.; Chen, H.; Zhang, J.; Liang, X.; Xiao, T. Butyrate ameliorates colorectal cancer through regulating intestinal microecological disorders. Anti-Cancer Drugs 2023, 34, 227–237.
- 46.Li, Y.; He, P.; Chen, Y.; Hu, J.; Deng, B.; Liu, C.; Dong, W. Microbial metabolite sodium butyrate enhances the anti-tumor efficacy of 5-fluorouracil against colorectal cancer by modulating PINK1/Parkin signaling and intestinal flora. Sci. Rep. 2024, 14, 13063.
- 47.Archer, S.Y.; Meng, S.; Shei, A.; Hodin, R.A. p21WAF1 is required for butyrate-mediated growth inhibition of human colon cancer cells. Proc. Natl. Acad. Sci. USA 1998, 95, 6791–6796.
- 48.Kruh, J. Effects of sodium butyrate, a new pharmacological agent, on cells in culture. Mol. Cell Biochem. 1981, 42, 65–82.
- 49.Chen, W.C.; Completo, G.C.; Sigal, D.S.; Crocker, P.R.; Saven, A.; Paulson, J.C. In vivo targeting of B-cell lymphoma with glycan ligands of CD22. Blood 2010, 115, 4778–4786.
- 50.Zaccai, N.R.; Maenaka, K.; Maenaka, T.; Crocker, P.R.; Brossmer, R.; Kelm, S.; Jones, E.Y. Structure-guided design of sialic acid-based Siglec inhibitors and crystallographic analysis in complex with sialoadhesin. Structure 2003, 11, 557–567.
- 51.Kelm, S.; Gerlach, J.; Brossmer, R.; Danzer, C.P.; Nitschke, L. The ligand-binding domain of CD22 is needed for inhibition of the B cell receptor signal, as demonstrated by a novel human CD22-specific inhibitor compound. J. Exp. Med. 2002, 195, 1207–1213.
- 52.Büll, C.; Boltje, T.J.; Wassink, M.; de Graaf, A.M.; van Delft, F.L.; den Brok, M.H.; Adema, G.J. Targeting aberrant sialylation in cancer cells using a fluorinated sialic acid analog impairs adhesion, migration, and in vivo tumor growth. Molecular cancer therapeutics. Mol. Cancer Ther. 2013, 12, 1935–1946.
- 53.Rodrigues, E.; Macauley, M. Hypersialylation in Cancer: Modulation of Inflammation and Therapeutic Opportunities. Cancers 2018, 10, 207.
- 54.Pearce, O.M.; Läubli, H. Sialic acids in cancer biology and immunity. Glycobiology 2015, 26, 111–128.
- 55.Hudak, J.E.; Canham, S.M.; Bertozzi, C.R. Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nat. Chem. Biol. 2014, 10, 69–75.
- 56.O’Reilly, M.K.; Paulson, J.C. Siglecs as targets for therapy in immune-cell-mediated disease. Trends in pharmacological sciences. Trends Pharmacol. Sci. 2009, 30, 240–248.
- 57.Jakobsche, C.E.; Parker, C.G.; Tao, R.N.; Kolesnikova, M.D.; Douglass, E.F., Jr.; Spiegel, D.A. Exploring binding and effector functions of natural human antibodies using synthetic immunomodulators. ACS Chem. Biol. 2013, 8, 2404–2411. https://doi.org/10.1021/cb4004942.
- 58.Murelli, R.P.; Zhang, A.X.; Michel, J.; Jorgensen, W.L.; Spiegel, D.A. Chemical control over immune recognition: A class of antibody-recruiting small molecules that target prostate cancer. J. Am. Chem. Soc. 2009, 131, 17090–17092. https://doi.org/10.1021/ja906844e.
- 59.Parker, C.G.; Domaoal, R.A.; Anderson, K.S.; Spiegel, D.A. An antibody-recruiting small molecule that targets HIV gp120. J. Am. Chem. Soc. 2009, 131, 16392–16394. https://doi.org/10.1021/ja9057647.
- 60.Fura, J.M.; Sabulski, M.J.; Pires, M.M. D-amino acid mediated recruitment of endogenous antibodies to bacterial surfaces. ACS Chem. Biol. 2014, 9, 1480–1489. https://doi.org/10.1021/cb5002685.
- 61.Cheadle, E.J.; Gornall, H.; Baldan, V.; Hanson, V.; Hawkins, R.E.; Gilham, D.E. CAR T cells: Driving the road from the laboratory to the clinic. Immunol. Rev. 2014, 257, 91–106. https://doi.org/10.1111/imr.12126.
- 62.Tomayko, M.M.; Reynolds, C.P. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother. Pharmacol. 1989, 24, 148–154.
How to Cite
Zhang, S.; Dong, H.; Wen, S.; Lin, Z.; Wu, X.; Han, J.; Han, S. A Sialic Acid-Caged Generic Platform for Sialoengineering of Tumors with Artificial Immuno-Ligand. Health and Metabolism 2025, 2 (3), 8. https://doi.org/10.53941/hm.2025.100023.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


