In vivo electrochemical biosensors (IVEBs) have emerged as pivotal tools in advancing personalized healthcare paradigms, particularly due to their exceptional capability for real-time tracking of dynamic physiological biomarkers. Their seamless integration into next-generation health monitoring platforms has not only revolutionized clinical diagnostics but also propelled the innovation of implantable sensing architectures, thereby redefining precision medicine strategies through continuous in situ bioanalytical measurements. This review highlights the latest advancements of IVEBs, including potentiometric, amperometric, and impedance biosensors, emphasizing their high sensitivity, specificity, and capability to function in complex biological environments. Additionally, this review discusses the limitations of current IVEBs, such as sensitivity, miniaturization, and applications of biodiversity. In future, researchers should use novel biocompatible nanomaterials and artificial intelligence algorithms to promote the development of IVEBs.
- Open Access
- Review
In Vivo Electrochemical Biosensors: Technology and Personalized Medicine Go Hand in Hand
- Yuxin Lou 1, 2, †,
- Siting Chen 1, 2, †,
- Shijin Peng 1, 2,
- Fei Lan 1, 2,
- Zhuowei Gao 3, 4,
- Ye Zhang 1, 2, *,
- Min Luo 1, 2, *
Author Information
Received: 24 Apr 2025 | Revised: 29 May 2025 | Accepted: 01 Jul 2025 | Published: 08 Aug 2025
Abstract
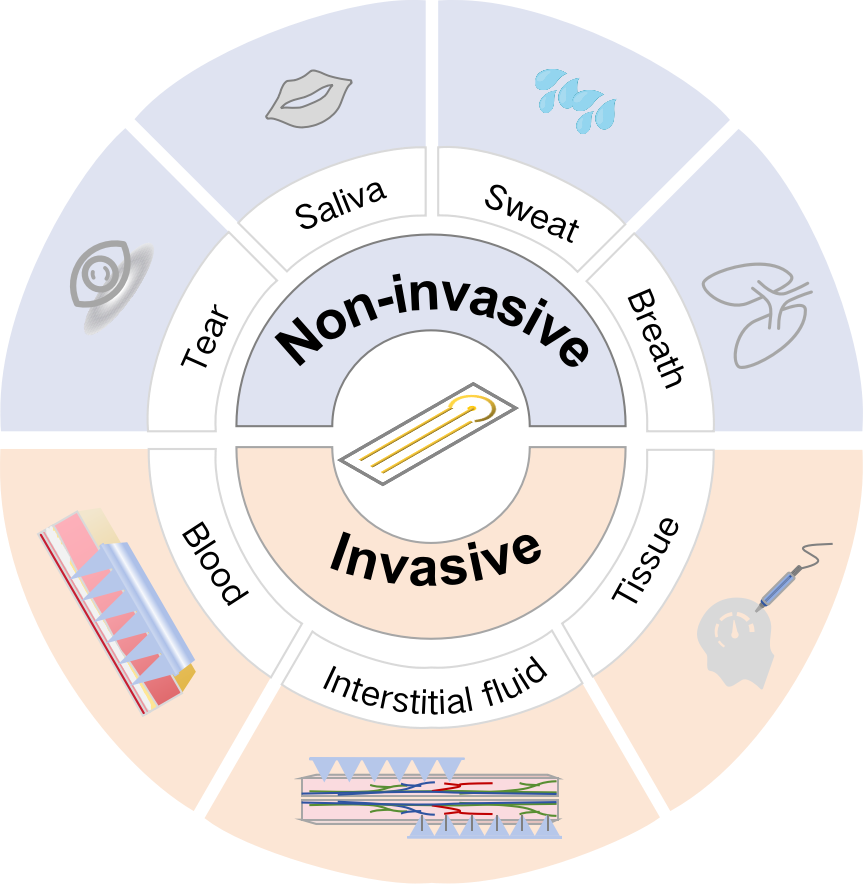
Graphical Abstract
References
- 1.Lan, T.; Zhang, J.; Lu, Y. Transforming the Blood Glucose Meter into a General Healthcare Meter for in Vitro Diagnostics in Mobile Health. Biotechnol. Adv. 2016, 34, 331–341. https://doi.org/10.1016/j.biotechadv.2016.03.002.
- 2.Duan, H.; Peng, S.; He, S.; Tang, S.; Goda, K.; Wang, C.H.; Li, M. Wearable Electrochemical Biosensors for Advanced Healthcare Monitoring. Adv. Sci. 2024, 12, 2411433. https://doi.org/10.1002/advs.202411433.
- 3.Christensen, K.; Doblhammer, G.; Rau, R.; Vaupel, J.W. Ageing Populations: The Challenges Ahead. Lancet Lond. Engl. 2009, 374, 1196–1208. https://doi.org/10.1016/S0140-6736(09)61460-4.
- 4.Chen, X.; Giles, J.; Yao, Y.; Yip, W.; Meng, Q.; Berkman, L.; Chen, H.; Chen, X.; Feng, J.; Feng, Z.; et al. The Path to Healthy Ageing in China: A Peking University–Lancet Commission. Lancet 2022, 400, 1967–2006. https://doi.org/10.1016/S0140-6736(22)01546-X.
- 5.Beard, J.R.; Officer, A.; de Carvalho, I.A.; Sadana, R.; Pot, A.M.; Michel, J.-P.; Lloyd-Sherlock, P.; Epping-Jordan, J.E.; Peeters, G.M.E.E.G.; Mahanani, W.R.; et al. The World Report on Ageing and Health: A Policy Framework for Healthy Ageing. Lancet Lond. Engl. 2016, 387, 2145–2154. https://doi.org/10.1016/S0140-6736(15)00516-4.
- 6.Ge, Y.; Taha, A.; Shah, S.A.; Dashtipour, K.; Zhu, S.; Cooper, J.; Abbasi, Q.H.; Imran, M.A. Contactless WiFi Sensing and Monitoring for Future Healthcare—Emerging Trends, Challenges, and Opportunities. IEEE Rev. Biomed. Eng. 2023, 16, 171–191. https://doi.org/10.1109/RBME.2022.3156810.
- 7.Iqbal, S.M.A.; Mahgoub, I.; Du, E.; Leavitt, M.A.; Asghar, W. Advances in Healthcare Wearable Devices. Npj Flex. Electron. 2021, 5, 9. https://doi.org/10.1038/s41528-021-00107-x.
- 8.Babu, M.; Lautman, Z.; Lin, X.; Sobota, M.H.B.; Snyder, M.P. Wearable Devices: Implications for Precision Medicine and the Future of Health Care. Annu. Rev. Med. 2024, 75, 401–415. https://doi.org/10.1146/annurev-med-052422-020437.
- 9.Tu, J.; Min, J.; Song, Y.; Xu, C.; Li, J.; Moore, J.; Hanson, J.; Hu, E.; Parimon, T.; Wang, T.-Y.; et al. A Wireless Patch for the Monitoring of C-Reactive Protein in Sweat. Nat. Biomed. Eng. 2023, 7, 1293–1306. https://doi.org/10.1038/s41551-023-01059-5.
- 10.Lin, T.; Xu, Y.; Zhao, A.; He, W.; Xiao, F. Flexible Electrochemical Sensors Integrated with Nanomaterials for in Situ Determination of Small Molecules in Biological Samples: A Review. Anal. Chim. Acta 2022, 1207, 339461. https://doi.org/10.1016/j.aca.2022.339461.
- 11.Pang, Y.; Yang, Z.; Yang, Y.; Ren, T. Wearable Electronics Based on 2D Materials for Human Physiological Information Detection. Small 2020, 16, 1901124. https://doi.org/10.1002/smll.201901124.
- 12.Mirzajani, H.; Abbasiasl, T.; Mirlou, F.; Istif, E.; Bathaei, M.J.; Dağ, Ç.; Deyneli, O.; Yazıcı, D.; Beker, L. An Ultra-Compact and Wireless Tag for Battery-Free Sweat Glucose Monitoring. Biosens. Bioelectron. 2022, 213, 114450. https://doi.org/10.1016/j.bios.2022.114450.
- 13.Chu, M.; Zhang, Y.; Ji, C.; Zhang, Y.; Yuan, Q.; Tan, J. DNA Nanomaterial-Based Electrochemical Biosensors for Clinical Diagnosis. ACS Nano 2024, 18, 31713–31736. https://doi.org/10.1021/acsnano.4c11857.
- 14.Zhang, R.; Belwal, T.; Li, L.; Lin, X.; Xu, Y.; Luo, Z. Nanomaterial-Based Biosensors for Sensing Key Foodborne Pathogens: Advances from Recent Decades. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1465–1487. https://doi.org/10.1111/1541-4337.12576.
- 15.Adeel, M.; Rahman, M.M.; Caligiuri, I.; Canzonieri, V.; Rizzolio, F.; Daniele, S. Recent Advances of Electrochemical and Optical Enzyme-Free Glucose Sensors Operating at Physiological Conditions. Biosens. Bioelectron. 2020, 165, 112331. https://doi.org/10.1016/j.bios.2020.112331.
- 16.Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully Integrated Wearable Sensor Arrays for Multiplexed in Situ Perspiration Analysis. Nature 2016, 529, 509–514. https://doi.org/10.1038/nature16521.
- 17.Laochai, T.; Yukird, J.; Promphet, N.; Qin, J.; Chailapakul, O.; Rodthongkum, N. Non-Invasive Electrochemical Immunosensor for Sweat Cortisol Based on L-Cys/AuNPs/ MXene Modified Thread Electrode. Biosens. Bioelectron. 2022, 203, 114039. https://doi.org/10.1016/j.bios.2022.114039.
- 18.Heller, A. Amperometric Biosensors. Curr. Opin. Biotechnol. 1996, 7, 50–54. https://doi.org/10.1016/S0958-1669(96)80094-2.
- 19.Hafeman, D.G.; Parce, J.W.; McConnell, H.M. Light-Addressable Potentiometric Sensor for Biochemical Systems. Science 1988, 240, 1182–1185. https://doi.org/10.1126/science.3375810.
- 20.Bahadır, E.B.; Sezgintürk, M.K. Electrochemical Biosensors for Hormone Analyses. Biosens. Bioelectron. 2015, 68, 62–71. https://doi.org/10.1016/j.bios.2014.12.054.
- 21.Wang, Y.; Xu, H.; Zhang, J.; Li, G. Electrochemical Sensors for Clinic Analysis. Sensors 2008, 8, 2043–2081. https://doi.org/10.3390/s8042043.
- 22.Wu, J.; Liu, H.; Chen, W.; Ma, B.; Ju, H. Device Integration of Electrochemical Biosensors. Nat. Rev. Bioeng. 2023, 1, 346–360. https://doi.org/10.1038/s44222-023-00032-w.
- 23.Karter, A.J.; Parker, M.M.; Moffet, H.H.; Gilliam, L.K.; Dlott, R. Association of Real-Time Continuous Glucose Monitoring with Glycemic Control and Acute Metabolic Events among Patients with Insulin-Treated Diabetes. JAMA 2021, 325, 1–12. https://doi.org/10.1001/jama.2021.6530.
- 24.Zeng, R.; Qiu, M.; Wan, Q.; Huang, Z.; Liu, X.; Tang, D.; Knopp, D. Smartphone-Based Electrochemical Immunoassay for Point-of-Care Detection of SARS-CoV-2 Nucleocapsid Protein. Anal. Chem. 2022, 94, 15155–15161. https://doi.org/10.1021/acs.analchem.2c03606.
- 25.Zhan, D.; Han, L.; Zhang, J.; He, Q.; Tian, Z.-W.; Tian, Z.-Q. Electrochemical Micro/Nano-Machining: Principles and Practices. Chem. Soc. Rev. 2017, 46, 1526–1544. https://doi.org/10.1039/C6CS00735J.
- 26.Li, S.; Ma, Q. Electrochemical Nano-Sensing Interface for Exosomes Analysis and Cancer Diagnosis. Biosens. Bioelectron. 2022, 214, 114554. https://doi.org/10.1016/j.bios.2022.114554.
- 27.Gao, F.; Liu, C.; Zhang, L.; Liu, T.; Wang, Z.; Song, Z.; Cai, H.; Fang, Z.; Chen, J.; Wang, J.; et al. Wearable and Flexible Electrochemical Sensors for Sweat Analysis: A Review. Microsyst. Nanoeng. 2023, 9, 1. https://doi.org/10.1038/s41378-022-00443-6.
- 28.La Belle, J.T.; Adams, A.; Lin, C.-E.; Engelschall, E.; Pratt, B.; Cook, C.B. Self-Monitoring of Tear Glucose: The Development of a Tear Based Glucose Sensor as an Alternative to Self-Monitoring of Blood Glucose. Chem. Commun. 2016, 52, 9197–9204. https://doi.org/10.1039/C6CC03609K.
- 29.Song, H.; Shin, H.; Seo, H.; Park, W.; Joo, B.J.; Kim, J.; Kim, J.; Kim, H.K.; Kim, J.; Park, J. Wireless Non-Invasive Monitoring of Cholesterol Using a Smart Contact Lens. Adv. Sci. 2022, 9, 2203597. https://doi.org/10.1002/advs.202203597.
- 30.Davis, N., Heikenfeld, J., Milla, C.; Javey, A. The Challenges and Promise of Sweat Sensing | Nature Biotechnology. Available online: https://www.nature.com/articles/s41587-023-02059-1 (accessed on 3 September 2024).
- 31.Zou, K.; Li, Q.; Li, D.; Jiao, Y.; Wang, L.; Li, L.; Wang, J.; Li, Y.; Gao, R.; Li, F.; et al. A Highly Selective Implantable Electrochemical Fiber Sensor for Real-Time Monitoring of Blood Homovanillic Acid. ACS Nano 2024, 18, 7485–7495. https://doi.org/10.1021/acsnano.3c11641.
- 32.Parrilla, M.; Detamornrat, U.; Domínguez-Robles, J.; Tunca, S.; Donnelly, R.F.; De Wael, K. Wearable Microneedle-Based Array Patches for Continuous Electrochemical Monitoring and Drug Delivery: Toward a Closed-Loop System for Methotrexate Treatment. ACS Sens. 2023, 8, 4161–4170. https://doi.org/10.1021/acssensors.3c01381.
- 33.Zhu, B.; Li, X.; Zhu, L.; Qi, M.; Cao, J.; Zhou, L.; Su, B. In Vivo Electrochemical Measurement of Glucose Variation in the Brain of Early Diabetic Mice. ACS Sens. 2023, 8, 4064–4070. https://doi.org/10.1021/acssensors.3c01165.
- 34.Zhu, X.; Ju, Y.; Chen, J.; Liu, D.; Liu, H. Nonenzymatic Wearable Sensor for Electrochemical Analysis of Perspiration Glucose. ACS Sens. 2018, 3, 1135–1141. https://doi.org/10.1021/acssensors.8b00168.
- 35.Mishra, R.K.; Sempionatto, J.R.; Li, Z.; Brown, C.; Galdino, N.M.; Shah, R.; Liu, S.; Hubble, L.J.; Bagot, K.; Tapert, S.; et al. Simultaneous Detection of Salivary Δ9-Tetrahydrocannabinol and Alcohol Using a Wearable Electrochemical Ring Sensor. Talanta 2020, 211, 120757. https://doi.org/10.1016/j.talanta.2020.120757.
- 36.Xue, Y.; Wu, F.; Zhao, X.; Ji, W.; Hou, L.; Yu, P.; Mao, L. Highly Sensitive Near-Field Electrochemical Sensor for In Vivo Monitoring of Respiratory Patterns. ACS Sens. 2024, 9, 2149–2155. https://doi.org/10.1021/acssensors.4c00261.
- 37.Yang, B.; Wang, H.; Kong, J.; Fang, X. Long-Term Monitoring of Ultratrace Nucleic Acids Using Tetrahedral Nanostructure-Based NgAgo on Wearable Microneedles. Nat. Commun. 2024, 15, 1936. https://doi.org/10.1038/s41467-024-46215-w.
- 38.Wang, M.; Yang, Y.; Min, J.; Song, Y.; Tu, J.; Mukasa, D.; Ye, C.; Xu, C.; Heflin, N.; McCune, J.S.; et al. A Wearable Electrochemical Biosensor for the Monitoring of Metabolites and Nutrients. Nat. Biomed. Eng. 2022, 6, 1225. https://doi.org/10.1038/s41551-022-00916-z.
- 39.Yoon, S.; Yoon, H.; Zahed, M.A.; Park, C.; Kim, D.; Park, J.Y. Multifunctional Hybrid Skin Patch for Wearable Smart Healthcare Applications. Biosens. Bioelectron. 2022, 196, 113685. https://doi.org/10.1016/j.bios.2021.113685.
- 40.Sankhala, D.; Sardesai, A.U.; Pali, M.; Lin, K.-C.; Jagannath, B.; Muthukumar, S.; Prasad, S. A Machine Learning-Based on-Demand Sweat Glucose Reporting Platform. Sci. Rep. 2022, 12, 2442. https://doi.org/10.1038/s41598-022-06434-x.
- 41.Luo, M.; Lan, F.; Yang, C.; Ji, T.; Lou, Y.; Zhu, Y.; Li, W.; Chen, S.; Gao, Z.; Luo, S.; et al. Sensitive Small Extracellular Vesicles Associated circRNAs Analysis Combined with Machine Learning for Precision Identification of Gastric Cancer. Chem. Eng. J. 2024, 491, 152094. https://doi.org/10.1016/j.cej.2024.152094.
- 42.Karimi-Maleh, H.; Orooji, Y.; Karimi, F.; Alizadeh, M.; Baghayeri, M.; Rouhi, J.; Tajik, S.; Beitollahi, H.; Agarwal, S.; Gupta, V.K.; et al. A Critical Review on the Use of Potentiometric Based Biosensors for Biomarkers Detection. Biosens. Bioelectron. 2021, 184, 113252. https://doi.org/10.1016/j.bios.2021.113252.
- 43.Kotanen, C.N.; Moussy, F.G.; Carrara, S.; Guiseppi-Elie, A. Implantable Enzyme Amperometric Biosensors. Biosens. Bioelectron. 2012, 35, 14–26. https://doi.org/10.1016/j.bios.2012.03.016.
- 44.Ma, S.; Wan, Z.; Wang, C.; Song, Z.; Ding, Y.; Zhang, D.; Chan, C.L.J.; Shu, L.; Huang, L.; Yang, Z.; et al. Ultra-Sensitive and Stable Multiplexed Biosensors Array in Fully Printed and Integrated Platforms for Reliable Perspiration Analysis. Adv. Mater. 2024, 36, 2311106. https://doi.org/10.1002/adma.202311106.
- 45.Labib, M.; Sargent, E.H.; Kelley, S.O. Electrochemical Methods for the Analysis of Clinically Relevant Biomolecules. Chem. Rev. 2016, 116, 9001–9090. https://doi.org/10.1021/acs.chemrev.6b00220.
- 46.Silva, B.V.M.; Cordeiro, M.T.; Rodrigues, M.A.B.; Marques, E.T.A.; Dutra, R.F. A Label and Probe-Free Zika Virus Immunosensor Prussian Blue@carbon Nanotube-Based for Amperometric Detection of the NS2B Protein. Biosensors 2021, 11, 157. https://doi.org/10.3390/bios11050157.
- 47.Wang, H.; Ma, Z. Copper Peroxide/ZIF-8 Self-Producing H2O2 Triggered Cascade Reaction for Amperometric Immunoassay of Carbohydrate Antigen 19-9. Biosens. Bioelectron. 2020, 169, 112644. https://doi.org/10.1016/j.bios.2020.112644.
- 48.Yao, Y.; Chen, J.; Guo, Y.; Lv, T.; Chen, Z.; Li, N.; Cao, S.; Chen, B.; Chen, T. Integration of Interstitial Fluid Extraction and Glucose Detection in One Device for Wearable Non-Invasive Blood Glucose Sensors. Biosens. Bioelectron. 2021, 179, 113078. https://doi.org/10.1016/j.bios.2021.113078.
- 49.Sehit, E.; Altintas, Z. Significance of Nanomaterials in Electrochemical Glucose Sensors: An Updated Review (2016–2020). Biosens. Bioelectron. 2020, 159, 112165. https://doi.org/10.1016/j.bios.2020.112165.
- 50.Engblom, S.O. The Phosphate Sensor. Biosens. Bioelectron. 1998, 13, 981–994. https://doi.org/10.1016/S0956-5663(98)00001-3.
- 51.Furst, A.L.; Francis, M.B. Impedance-Based Detection of Bacteria. Chem. Rev. 2019, 119, 700–726. https://doi.org/10.1021/acs.chemrev.8b00381.
- 52.Zhou, J.; Zhou, S.; Fan, P.; Li, X.; Ying, Y.; Ping, J.; Pan, Y. Implantable Electrochemical Microsensors for In Vivo Monitoring of Animal Physiological Information. Nano-Micro Lett. 2024, 16, 49. https://doi.org/10.1007/s40820-023-01274-4.
- 53.Bonafè, F.; Decataldo, F.; Zironi, I.; Remondini, D.; Cramer, T.; Fraboni, B. AC Amplification Gain in Organic Electrochemical Transistors for Impedance-Based Single Cell Sensors. Nat. Commun. 2022, 13, 5423. https://doi.org/10.1038/s41467-022-33094-2.
- 54.Devarakonda, S.; Ganapathysubramanian, B.; Shrotriya, P. Impedance-Based Nanoporous Anodized Alumina/ITO Platforms for Label-Free Biosensors. ACS Appl. Mater. Interfaces 2022, 14, 150–158. https://doi.org/10.1021/acsami.1c17243.
- 55.Strong, M.E.; Richards, J.R.; Torres, M.; Beck, C.M.; La Belle, J.T. Faradaic Electrochemical Impedance Spectroscopy for Enhanced Analyte Detection in Diagnostics. Biosens. Bioelectron. 2021, 177, 112949. https://doi.org/10.1016/j.bios.2020.112949.
- 56.Balakrishnan, G.; Bhat, A.; Naik, D.; Kim, J.S.; Marukyan, S.; Gido, L.; Ritter, M.; Khair, A.S.; Bettinger, C.J. Gelatin-Based Ingestible Impedance Sensor to Evaluate Gastrointestinal Epithelial Barriers. Adv. Mater. 2023, 35, 2211581. https://doi.org/10.1002/adma.202211581.
- 57.Abdul Ghani, M.A.; Nordin, A.N.; Zulhairee, M.; Che Mohamad Nor, A.; Shihabuddin Ahmad Noorden, M.; Muhamad Atan, M.K.F.; Ab Rahim, R.; Mohd Zain, Z. Portable Electrochemical Biosensors Based on Microcontrollers for Detection of Viruses: A Review. Biosensors 2022, 12, 666. https://doi.org/10.3390/bios12080666.
- 58.Fedor, S.; Lewis, R.; Pedrelli, P.; Mischoulon, D.; Curtiss, J.; Picard, R.W. Wearable Technology in Clinical Practice for Depressive Disorder. N. Engl. J. Med. 2023, 389, 2457–2466. https://doi.org/10.1056/NEJMra2215898.
- 59.Chen, X.; Kim, D.-H.; Lu, N. Introduction: Wearable Devices. Chem. Rev. 2024, 124, 6145–6147. https://doi.org/10.1021/acs.chemrev.4c00271.
- 60.Min, J.; Sempionatto, J.R.; Teymourian, H.; Wang, J.; Gao, W. Wearable Electrochemical Biosensors in North America. Biosens. Bioelectron. 2021, 172, 112750. https://doi.org/10.1016/j.bios.2020.112750.
- 61.Promphet, N.; Ummartyotin, S.; Ngeontae, W.; Puthongkham, P.; Rodthongkum, N. Non-Invasive Wearable Chemical Sensors in Real-Life Applications. Anal. Chim. Acta 2021, 1179, 338643. https://doi.org/10.1016/j.aca.2021.338643.
- 62.Tian, H.; Ma, J.; Li, Y.; Xiao, X.; Zhang, M.; Wang, H.; Zhu, N.; Hou, C.; Ulstrup, J. Electrochemical Sensing Fibers for Wearable Health Monitoring Devices. Biosens. Bioelectron. 2024, 246, 115890. https://doi.org/10.1016/j.bios.2023.115890.
- 63.Huang, J.; Wang, H.; Wu, Q.; Yin, J.; Zhou, H.; He, Y. Clinical Research on Neurological and Psychiatric Diagnosis and Monitoring Using Wearable Devices: A Literature Review. Interdiscip. Med. 2024, 2, e20230037. https://doi.org/10.1002/INMD.20230037.
- 64.Vaddiraju, S.; Burgess, D.J.; Tomazos, I.; Jain, F.C.; Papadimitrakopoulos, F. Technologies for Continuous Glucose Monitoring: Current Problems and Future Promises. J. Diabetes Sci. Technol. 2010, 4, 1540–1562. https://doi.org/10.1177/193229681000400632.
- 65.Regiart, M.; Ledo, A.; Fernandes, E.; Messina, G.A.; Brett, C.M.A.; Bertotti, M.; Barbosa, R.M. Highly Sensitive and Selective Nanostructured Microbiosensors for Glucose and Lactate Simultaneous Measurements in Blood Serum and in Vivo in Brain Tissue. Biosens. Bioelectron. 2022, 199, 113874. https://doi.org/10.1016/j.bios.2021.113874.
- 66.Liu, W.-T.; Cao, Y.-P.; Zhou, X.-H.; Han, D. Interstitial Fluid Behavior and Diseases. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2022, 9, e2100617. https://doi.org/10.1002/advs.202100617.
- 67.Bakhshandeh, F.; Zheng, H.; Barra, N.G.; Sadeghzadeh, S.; Ausri, I.; Sen, P.; Keyvani, F.; Rahman, F.; Quadrilatero, J.; Liu, J.; et al. Wearable Aptalyzer Integrates Microneedle and Electrochemical Sensing for In Vivo Monitoring of Glucose and Lactate in Live Animals. Adv. Mater. 2024, 36, 2313743. https://doi.org/10.1002/adma.202313743.
- 68.Li, J.; Wei, M.; Gao, B. A Review of Recent Advances in Microneedle-Based Sensing within the Dermal ISF That Could Transform Medical Testing. ACS Sens. 2024, 9, 1149–1161. https://doi.org/10.1021/acssensors.4c00142.
- 69.Parrilla, M.; Detamornrat, U.; Domínguez-Robles, J.; Donnelly, R.F.; De Wael, K. Wearable Hollow Microneedle Sensing Patches for the Transdermal Electrochemical Monitoring of Glucose. Talanta 2022, 249, 123695. https://doi.org/10.1016/j.talanta.2022.123695.
- 70.Monteiro, T.; Dias, C.; Lourenço, C.F.; Ledo, A.; Barbosa, R.M.; Almeida, M.G. Microelectrode Sensor for Real-Time Measurements of Nitrite in the Living Brain, in the Presence of Ascorbate. Biosensors 2021, 11, 277. https://doi.org/10.3390/bios11080277.
- 71.Bandodkar, A.J.; Wang, J. Non-Invasive Wearable Electrochemical Sensors: A Review. Trends Biotechnol. 2014, 32, 363–371. https://doi.org/10.1016/j.tibtech.2014.04.005.
- 72.Peng, H.-L.; Zhang, Y.; Liu, H.; Gao, C. Flexible Wearable Electrochemical Sensors Based on AuNR/PEDOT:PSS for Simultaneous Monitoring of Levodopa and Uric Acid in Sweat. ACS Sens. 2024, 9, 3296–3306. https://doi.org/10.1021/acssensors.4c00649.
- 73.Shahub, S.; Lin, K.-C.; Muthukumar, S.; Prasad, S. A Proof-of-Concept Electrochemical Skin Sensor for Simultaneous Measurement of Glial Fibrillary Acidic Protein (GFAP) and Interleukin-6 (IL-6) for Management of Traumatic Brain Injuries. Biosensors 2022, 12, 1095. https://doi.org/10.3390/bios12121095.
- 74.Tonyushkina, K.; Nichols, J.H. Glucose Meters: A Review of Technical Challenges to Obtaining Accurate Results. J. Diabetes Sci. Technol. 2009, 3, 971–980. https://doi.org/10.1177/193229680900300446.
- 75.Clark, L.C.; Lyons, C. ELECTRODE SYSTEMS FOR CONTINUOUS MONITORING IN CARDIOVASCULAR SURGERY. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. https://doi.org/10.1111/j.1749-6632.1962.tb13623.x.
- 76.Karon, B.S.; Boyd, J.C.; Klee, G.G. Glucose Meter Performance Criteria for Tight Glycemic Control Estimated by Simulation Modeling. Clin. Chem. 2010, 56, 1091–1097. https://doi.org/10.1373/clinchem.2010.145367.
- 77.Villena Gonzales, W.; Mobashsher, A.; Abbosh, A. The Progress of Glucose Monitoring—A Review of Invasive to Minimally and Non-Invasive Techniques, Devices and Sensors. Sensors 2019, 19, 800. https://doi.org/10.3390/s19040800.
- 78.Emaminejad, S.; Gao, W.; Wu, E.; Davies, Z.A.; Yin Yin Nyein, H.; Challa, S.; Ryan, S.P.; Fahad, H.M.; Chen, K.; Shahpar, Z.; et al. Autonomous Sweat Extraction and Analysis Applied to Cystic Fibrosis and Glucose Monitoring Using a Fully Integrated Wearable Platform. Proc. Natl. Acad. Sci. USA 2017, 114, 4625–4630. https://doi.org/10.1073/pnas.1701740114.
- 79.Hong, X.; Wu, H.; Wang, C.; Zhang, X.; Wei, C.; Xu, Z.; Chen, D.; Huang, X. Hybrid Janus Membrane with Dual-Asymmetry Integration of Wettability and Conductivity for Ultra-Low-Volume Sweat Sensing. ACS Appl. Mater. Interfaces 2022, 14, 9644–9654. https://doi.org/10.1021/acsami.1c16820.
- 80.Pundir, C.S.; Narwal, V.; Batra, B. Determination of Lactic Acid with Special Emphasis on Biosensing Methods: A Review. Biosens. Bioelectron. 2016, 86, 777–790. https://doi.org/10.1016/j.bios.2016.07.076.
- 81.Saha, T.; Fang, J.; Yokus, M.A.; Mukherjee, S.; Bozkurt, A.; Daniele, M.A.; Dickey, M.D.; Velev, O.D. A Wearable Patch for Prolonged Sweat Lactate Harvesting and Sensing. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Online, 1–5 November 2021; pp. 6863–6866. https://doi.org/10.1109/EMBC46164.2021.9630881.
- 82.Saha, T.; Songkakul, T.; Knisely, C.T.; Yokus, M.A.; Daniele, M.A.; Dickey, M.D.; Bozkurt, A.; Velev, O.D. Wireless Wearable Electrochemical Sensing Platform with Zero-Power Osmotic Sweat Extraction for Continuous Lactate Monitoring. ACS Sens. 2022, 7, 2037–2048. https://doi.org/10.1021/acssensors.2c00830.
- 83.Niu, J.; Lin, S.; Chen, D.; Wang, Z.; Cao, C.; Gao, A.; Cui, S.; Liu, Y.; Hong, Y.; Zhi, X.; et al. A Fully Elastic Wearable Electrochemical Sweat Detection System of Tree-Bionic Microfluidic Structure for Real-Time Monitoring. Small 2024, 20, 2306769. https://doi.org/10.1002/smll.202306769.
- 84.Zhang, X.; Tang, Y.; Wu, H.; Wang, Y.; Niu, L.; Li, F. Integrated Aptasensor Array for Sweat Drug Analysis. Anal. Chem. 2022, 94, 7936–7943. https://doi.org/10.1021/acs.analchem.2c00736.
- 85.Zhang, X.; Zhang, J.; Cai, Y.; Xu, S.; Wu, H.; Chen, X.; Huang, Y.; Li, F. Integrated Electrochemical Aptasensor Array toward Monitoring Anticancer Drugs in Sweat. Anal. Chem. 2024, 96, 4997–5005. https://doi.org/10.1021/acs.analchem.4c00297.
- 86.Koch, C.; Reilly-O’Donnell, B.; Gutierrez, R.; Lucarelli, C.; Ng, F.S.; Gorelik, J.; Ivanov, A.P.; Edel, J.B. Nanopore Sequencing of DNA-Barcoded Probes for Highly Multiplexed Detection of microRNA, Proteins and Small Biomarkers. Nat. Nanotechnol. 2023, 18, 1483–1491. https://doi.org/10.1038/s41565-023-01479-z.
- 87.Chen, R.; Du, X.; Cui, Y.; Zhang, X.; Ge, Q.; Dong, J.; Zhao, X. Vertical Flow Assay for Inflammatory Biomarkers Based on Nanofluidic Channel Array and SERS Nanotags. Small 2020, 16, 2002801. https://doi.org/10.1002/smll.202002801.
- 88.Tanak, A.S.; Muthukumar, S.; Krishnan, S.; Schully, K.L.; Clark, D.V.; Prasad, S. Multiplexed Cytokine Detection Using Electrochemical Point-of-Care Sensing Device towards Rapid Sepsis Endotyping. Biosens. Bioelectron. 2021, 171, 112726. https://doi.org/10.1016/j.bios.2020.112726.
- 89.Zheng, L.; Zhu, D.; Xiao, Y.; Zheng, X.; Chen, P. Microneedle Coupled Epidermal Sensor for Multiplexed Electrochemical Detection of Kidney Disease Biomarkers. Biosens. Bioelectron. 2023, 237, 115506. https://doi.org/10.1016/j.bios.2023.115506.
- 90.Wang, J.; Wang, L.; Li, G.; Yan, D.; Liu, C.; Xu, T.; Zhang, X. Ultra-Small Wearable Flexible Biosensor for Continuous Sweat Analysis. ACS Sens. 2022, 7, 3102–3107. https://doi.org/10.1021/acssensors.2c01533.
- 91.Lu, X.; Zhou, X.; Song, B.; Zhang, H.; Cheng, M.; Zhu, X.; Wu, Y.; Shi, H.; Chu, B.; He, Y.; et al. Framework Nucleic Acids Combined with 3D Hybridization Chain Reaction Amplifiers for Monitoring Multiple Human Tear Cytokines. Adv. Mater. 2024, 36, 2400622. https://doi.org/10.1002/adma.202400622.
- 92.Aihara, M.; Kubota, N.; Minami, T.; Shirakawa, R.; Sakurai, Y.; Hayashi, T.; Iwamoto, M.; Takamoto, I.; Kubota, T.; Suzuki, R.; et al. Association between Tear and Blood Glucose Concentrations: Random Intercept Model Adjusted with Confounders in Tear Samples Negative for Occult Blood. J. Diabetes Investig. 2021, 12, 266–276. https://doi.org/10.1111/jdi.13344.
- 93.Li, J.; Yu, H.; Zhao, J.; Qiao, X.; Chen, X.; Lu, Z.; Li, Q.; Lin, H.; Wu, W.; Zeng, W.; et al. Metal–Organic Framework-Based Surface-Enhanced Raman Scattering Sensing Platform for Trace Malondialdehyde Detection in Tears. Nano Lett. 2024, 24, 7792–7799. https://doi.org/10.1021/acs.nanolett.4c01978.
- 94.Park, W.; Seo, H.; Kim, J.; Hong, Y.-M.; Song, H.; Joo, B.J.; Kim, S.; Kim, E.; Yae, C.-G.; Kim, J.; et al. In-Depth Correlation Analysis between Tear Glucose and Blood Glucose Using a Wireless Smart Contact Lens. Nat. Commun. 2024, 15, 2828. https://doi.org/10.1038/s41467-024-47123-9.
- 95.Liao, C.; Chen, X.; Fu, Y. Salivary Analysis: An Emerging Paradigm for Non-Invasive Healthcare Diagnosis and Monitoring. Interdiscip. Med. 2023, 1, e20230009. https://doi.org/10.1002/INMD.20230009.
- 96.Zhang, C.-Z.; Cheng, X.-Q.; Li, J.-Y.; Zhang, P.; Yi, P.; Xu, X.; Zhou, X.-D. Saliva in the Diagnosis of Diseases. Int. J. Oral Sci. 2016, 8, 133–137. https://doi.org/10.1038/ijos.2016.38.
- 97.Song, M.; Bai, H.; Zhang, P.; Zhou, X.; Ying, B. Promising Applications of Human-Derived Saliva Biomarker Testing in Clinical Diagnostics. Int. J. Oral Sci. 2023, 15, 1–17. https://doi.org/10.1038/s41368-022-00209-w.
- 98.Kim, J.; Imani, S.; de Araujo, W.R.; Warchall, J.; Valdés-Ramírez, G.; Paixão, T.R.L.C.; Mercier, P.P.; Wang, J. Wearable Salivary Uric Acid Mouthguard Biosensor with Integrated Wireless Electronics. Biosens. Bioelectron. 2015, 74, 1061–1068. https://doi.org/10.1016/j.bios.2015.07.039.
- 99.Lim, H.-R.; Lee, S.M.; Park, S.; Choi, C.; Kim, H.; Kim, J.; Mahmood, M.; Lee, Y.; Kim, J.-H.; Yeo, W.-H. Smart Bioelectronic Pacifier for Real-Time Continuous Monitoring of Salivary Electrolytes. Biosens. Bioelectron. 2022, 210, 114329. https://doi.org/10.1016/j.bios.2022.114329.
- 100.Jin, X.; Zha, L.; Wang, F.; Wang, Y.; Zhang, X. Fully Integrated Wearable Humidity Sensor for Respiration Monitoring. Front. Bioeng. Biotechnol. 2022, 10, 1070855.
- 101.Choi, S.; Yu, H.; Jang, J.; Kim, M.; Kim, S.; Jeong, H.S.; Kim, I. Nitrogen-Doped Single Graphene Fiber with Platinum Water Dissociation Catalyst for Wearable Humidity Sensor. Small 2018, 14, 1703934. https://doi.org/10.1002/smll.201703934.
- 102.Wang, C.; Cai, Y.; Zhou, W.; Chen, P.; Xu, L.; Han, T.; Hu, Y.; Xu, X.; Liu, B.; Yu, X. A Wearable Respiration Sensor for Real-Time Monitoring of Chronic Kidney Disease. ACS Appl. Mater. Interfaces 2022, 14, 12630–12639. https://doi.org/10.1021/acsami.1c23878.
- 103.Ahmed, A.; Shahzad, A.; Naseem, A.; Ali, S.; Ahmad, I. Evaluating the effectiveness of data governance frameworks in ensuring security and privacy of healthcare data: A quantitative analysis of ISO standards, GDPR, and HIPAA in blockchain technology. PloS ONE 2025, 20, e0324285. https://doi.org/10.1371/journal.pone.0324285.
How to Cite
Lou, Y.; Chen, S.; Peng, S.; Lan, F.; Gao, Z.; Zhang, Y.; Luo, M. In Vivo Electrochemical Biosensors: Technology and Personalized Medicine Go Hand in Hand. Health and Metabolism 2025, 2 (4), 2. https://doi.org/10.53941/hm.2025.100025.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


