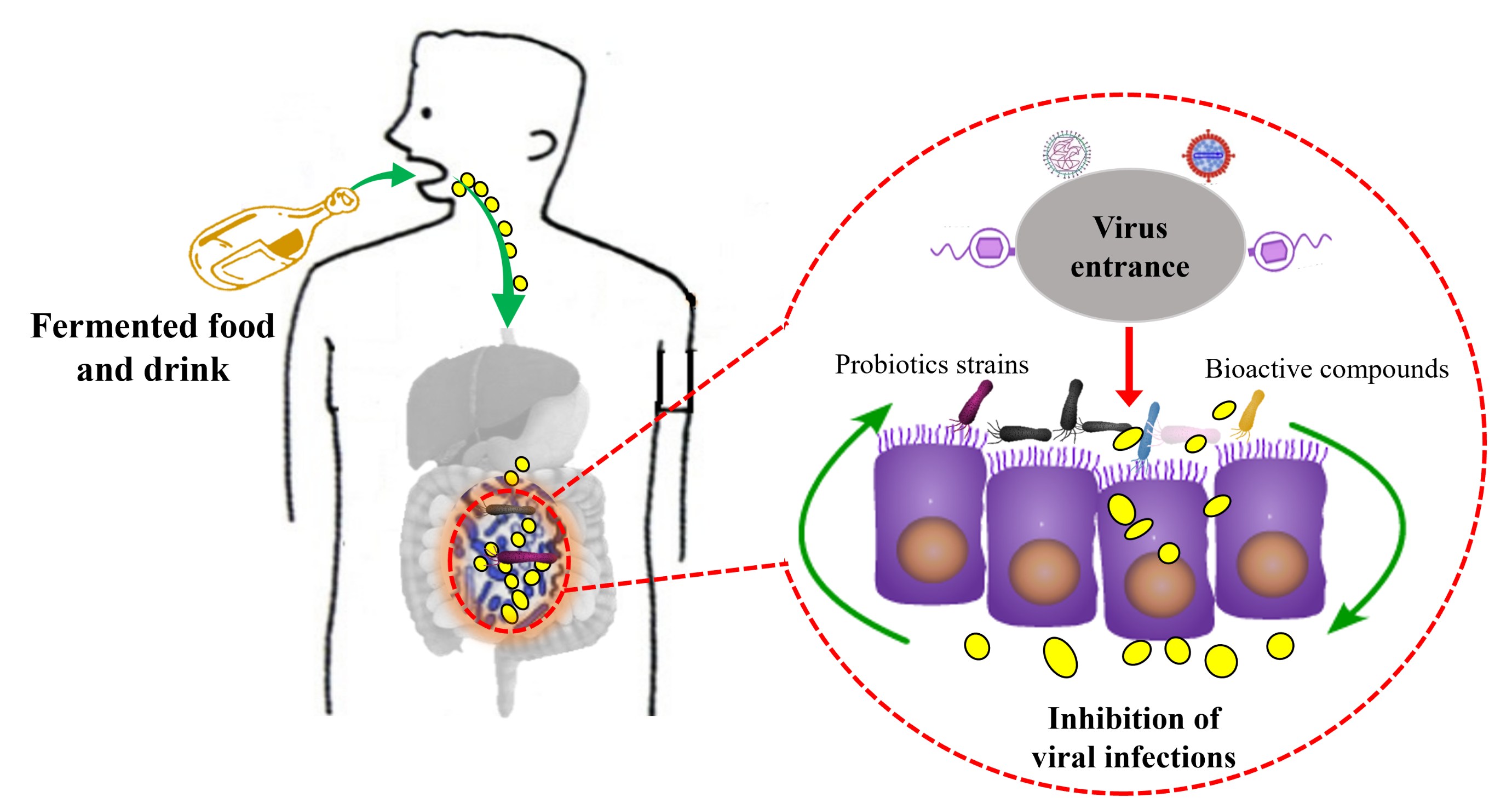
Fermented foods have been shown to exert positive effects on gut health and immune function. However, the potential of fermented foods to enhance the bioavailability of bioactive compounds and support the growth of the beneficial microbial community’s key factors in antiviral immunity remains less explored. In this review, we show that probiotic-fermented food improves the bioactive compound contents and is increasingly studied by basic and clinical researchers. Bioactive compounds, including phenolic, alkaloids, terpenoids, flavonoids, stilbenes, coumarins, tannins, anthocyanidins, flavones, isoflavonoids, and polyphenols, are increased in the probiotic fermentation conditions. Additionally, beneficial bacteria such as Lactobacilli, Bifidobacteria, Pediococcus, and Weissella are also restored in the fermented foods. These bioactive compounds, combined with a functional microbiota, play a role in preventing viral infections by targeting influenza, noroviruses (NoVs), Murine norovirus-1 (MNV-1), and COVID-19, while also stimulating the immune function of the host. It was suggested that clinical and pre-clinical investigations are required to explore the dose-response and duration efficacy of probiotic fermented foods against viral infections.
- Open Access
- Review
Fermented Foods Strengthen Immunity against Viral Infections
- Xiaoqian Zhou 1,
- Binyu Cui 1,
- Xiaoyu Wang 2,
- Aman Khan 1, *,
- Weidong Wang 1, *
Author Information
Received: 28 Apr 2025 | Revised: 21 May 2025 | Accepted: 12 Jun 2025 | Published: 12 Aug 2025
Abstract
Graphical Abstract
References
- 1.Lei, G.; Khan, A.; Budryn, G.; Grzelczyk, J. Probiotic products from laboratory to commercialization. Trends Food Sci. Technol. 2025, 155, 104807 https://doi.org/10.1016/j.tifs.2024.104807.
- 2.Hoxha, R.; Todorov, D.; Hinkov, A.; Shishkova, K.; Evstatieva, Y.; Nikolova, D. In Vitro Screening of Antiviral Activity of Lactic Acid Bacteria Isolated from Traditional Fermented Foods. Microbiol. Res. 2023, 14, 333–342. https://doi.org/10.3390/microbiolres14010026.
- 3.Varsha, K.K.; Narisetty, V.; Brar, K.K.; Madhavan, A.; Alphy, M.P.; Sindhu, R.; Awasthi, M.K.; Varjani, S.; Binod, P. Bioactive metabolites in functional and fermented foods and their role as immunity booster and anti-viral innate mechanisms. J. Food Sci. Technol. 2022, 60, 2309–2318. https://doi.org/10.1007/s13197-022-05528-8.
- 4.Khan, A.; Wang, W.; Ji, J.; Ling, Z.; Liu, P.; Xiao, S.; Han, H.; Salama, E.S.; Kumar Khanal, S.; Li, X. Fermented lily bulbs by Jiangshui probiotics improves lung health in mice. Food Chem. 2023, 440, 138270. https://doi.org/10.1016/j.foodchem.2023.138270.
- 5.Intharuksa, A.; Kuljarusnont, S.; Sasaki, Y.; Tungmunnithum, D. Flavonoids and Other Polyphenols: Bioactive Molecules from Traditional Medicine Recipes/Medicinal Plants and Their Potential for Phytopharmaceutical and Medical Application. Molecules 2024, 29, 5760 https://doi.org/10.3390/molecules29235760.
- 6.Özpınar, F.B.; İspirli, H.; Kayacan, S.; Korkmaz, K.; Dere, S.; Sagdic, O.; Alkay, Z.; Tunçil, Y.E.; Ayyash, M.; Dertli, E. Physicochemical and structural characterisation of a branched dextran type exopolysaccharide (EPS) from Weissella confusa S6 isolated from fermented sausage (Sucuk). Int. J. Biol. Macromol. 2024, 264, 130507. https://doi.org/10.1016/j.ijbiomac.2024.130507.
- 7.Zheng, Z.; Xie, G.; Liu, H.; Tan, G.; Li, L.; Liu, W.; Li, M. Fermented ginseng leaf enriched with rare ginsenosides relieves exercise-induced fatigue via regulating metabolites of muscular interstitial fluid, satellite cells-mediated muscle repair and gut microbiota. J. Funct. Foods 2021, 83, 104509. https://doi.org/10.1016/j.jff.2021.104509.
- 8.Zhu, C.; Guan, Q.; Song, C.; Zhong, L.; Ding, X.; Zeng, H.; Nie, P.; Song, L. Regulatory effects of Lactobacillus fermented black barley on intestinal microbiota of NAFLD rats. Food Res. Int. 2021, 147, 110467. https://doi.org/10.1016/j.foodres.2021.110467.
- 9.Zhao, Y.; Wu, C.; Zhu, Y.; Zhou, C.; Xiong, Z.; Samy Eweys, A.; Zhou, H.; Dong, Y.; Xiao, X. Metabolomics strategy for revealing the components in fermented barley extracts with Lactobacillus plantarum dy-1. Food Res. Int. 2021, 139, 109808. https://doi.org/10.1016/j.foodres.2020.109808.
- 10.Peruzzolo, M.; Ceni, G.C.; Junges, A.; Zeni, J.; Cansian, R.L.; Backes, G.T. Probiotics: Health benefits, microencapsulation, and viability, combination with natural compounds, and applications in foods. Food Biosci. 2025, 66, 106253. https://doi.org/10.1016/j.fbio.2025.106253.
- 11.Sakandar, H.A.; Zhang, H. Trends in Probiotic(s)-Fermented milks and their in vivo functionality: A review. Trends Food Sci. Technol. 2021, 110, 55–65. https://doi.org/10.1016/j.tifs.2021.01.054.
- 12.Muhialdin, B.J.; Kadum, H.; Meor Hussin, A.S. Metabolomics profiling of fermented cantaloupe juice and the potential application to extend the shelf life of fresh cantaloupe juice for six months at 8 °C. Food Control 2021, 120, 107555. https://doi.org/10.1016/j.foodcont.2020.107555.
- 13.Wan-Mohtar, W.A.A.Q.I.; Ilham, Z.; Jamaludin, A.A.; David, W.; Mohd Zaini, N.A. Fermented foods as alternative functional foods during post-pandemic in Asia. Front. Food Sci. Technol. 2022, 2, 1047970. https://doi.org/10.3389/frfst.2022.1047970.
- 14.Wen-qiong, W.; Jie-long, Z.; Qian, Y.; Ji-yang, Z.; Mao-lin, L.; Rui-xia, G.; Yujun, H. Structural and compositional changes of whey protein and blueberry juice fermented using Lactobacillus plantarum or Lactobacillus casei during fermentation. RSC Adv. 2021, 11, 26291–26302. https://doi.org/10.1039/d1ra04140a.
- 15.Kayacan Çakmakoğlu, S.; Dere, S.; Beki̇roğlu, H.; Bozkurt, F.; Karasu, S.; Dertli̇, E.; Türker, M.; Sagdic, O. Production of bioactive peptides during yogurt fermentation, their extraction and functional characterization. Food Biosci. 2024, 61, 104805. https://doi.org/10.1016/j.fbio.2024.104805.
- 16.Wei, J.; Zhang, Y.; Li, H.; Wang, F.; Yao, S. Toll-like receptor 4: A potential therapeutic target for multiple human diseases. Biomed. Pharmacother. 2023, 166, 115338. https://doi.org/10.1016/j.biopha.2023.115338.
- 17.de Araújo, F.F.; Farias, D.P. Psychobiotics: An emerging alternative to ensure mental health amid the COVID-19 outbreak? Trends Food Sci. Technol. 2020, 103, 386–387. https://doi.org/10.1016/j.tifs.2020.07.006.
- 18.Seo, D.J.; Jung, D.; Jung, S.; Yeo, D.; Choi, C. Inhibitory effect of lactic acid bacteria isolated from kimchi against murine norovirus. Food Control 2020, 109, 106881. https://doi.org/10.1016/j.foodcont.2019.106881.
- 19.Starosila, D.; Rybalko, S.; Varbanetz, L.; Ivanskaya, N.; Sorokulova, I. Anti-influenza Activity of a Bacillus subtilis Probiotic Strain. Antimicrob. Agents Chemother. 2017, 61, 00539–17. https://doi.org/10.1128/aac.00539-17.
- 20.Tonucci, L.B.; dos Santos Olbrich, K.M.; de Oliveira Licursi, L.; Rocha Ribeiro, S.M.; Duarte Martino, H.S. Clinical application of probiotics in type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. Clin. Nutr. 2017, 36, 85–92. https://doi.org/10.1016/j.clnu.2015.11.011.
- 21.Mirashrafi, S.; Moravejolahkami, A.R.; Balouch Zehi, Z.; Hojjati Kermani, M.A.; Bahreini-Esfahani, N.; Haratian, M.; Ganjali Dashti, M.; Pourhossein, M. The efficacy of probiotics on virus titres and antibody production in virus diseases: A systematic review on recent evidence for COVID-19 treatment. Clin. Nutr. ESPEN 2021, 46, 1–8. https://doi.org/10.1016/j.clnesp.2021.10.016.
- 22.Hamida, R.S.; Shami, A.; Ali, M.A.; Almohawes, Z.N.; Mohammed, A.E.; Bin-Meferij, M.M. Kefir: A protective dietary supplementation against viral infection. Biomed. Pharmacother. 2021, 133, 110974. https://doi.org/10.1016/j.biopha.2020.110974.
- 23.Tomas, M.; Capanoglu, E.; Bahrami, A.; Hosseini, H.; Akbari‐Alavijeh, S.; Shaddel, R.; Rehman, A.; Rezaei, A.; Rashidinejad, A.; Garavand, F.; et al. The direct and indirect effects of bioactive compounds against coronavirus. Food Front. 2021, 3, 96–123. https://doi.org/10.1002/fft2.119.
- 24.Wang, S.; Liu, X.; Tamura, T.; Kyouno, N.; Zhang, H.; Chen, J.Y. Effect of volatile compounds on the quality of miso (traditional Japanese fermented soybean paste). Lwt 2021, 139, 110573. https://doi.org/10.1016/j.lwt.2020.110573.
- 25.Wu, Y.; Ye, Z.; Feng, P.; Li, R.; Chen, X.; Tian, X.; Han, R.; Kakade, A.; Liu, P.; Li, X. Limosilactobacillus fermentum JL-3 isolated from “Jiangshui” ameliorates hyperuricemia by degrading uric acid. Gut Microbes 2021, 13, 1897211. https://doi.org/10.1080/19490976.2021.1897211.
- 26.Kim, N.; Lee, J.; Song, H.S.; Oh, Y.J.; Kwon, M.-S.; Yun, M.; Lim, S.K.; Park, H.K.; Jang, Y.S.; Lee, S.; et al. Kimchi intake alleviates obesity-induced neuroinflammation by modulating the gut-brain axis. Food Res. Int. 2022, 158, 111533. https://doi.org/10.1016/j.foodres.2022.111533.
- 27.Tamang, J.P.; Anupma, A.; Nakibapher Jones Shangpliang, H. Ethno-microbiology of Tempe, an Indonesian fungal-fermented soybean food and Koji, a Japanese fungal starter culture. Curr. Opin. Food Sci. 2022, 48, 100912. https://doi.org/10.1016/j.cofs.2022.100912.
- 28.Hinojosa-Avila, C.R.; García-Gamboa, R.; Chedraui-Urrea, J.J.T.; García-Cayuela, T. Exploring the potential of probiotic-enriched beer: Microorganisms, fermentation strategies, sensory attributes, and health implications. Food Res. Int. 2024, 175, 113717. https://doi.org/10.1016/j.foodres.2023.113717.
- 29.Grom, L.C.; Coutinho, N.M.; Guimarães, J.T.; Balthazar, C.F.; Silva, R.; Rocha, R.S.; Freitas, M.Q.; Duarte, M.C.K.H.; Pimentel, T.C.; Esmerino, E.A.; et al. Probiotic dairy foods and postprandial glycemia: A mini-review. Trends Food Sci. Technol. 2020, 101, 165–171. https://doi.org/10.1016/j.tifs.2020.05.012.
- 30.Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented Foods as a Dietary Source of Live Organisms. Front. Microbiol. 2018, 9, 1785. https://doi.org/10.3389/fmicb.2018.01785.
- 31.Zhang, T.; Geng, S.; Cheng, T.; Mao, K.; Chitrakar, B.; Gao, J.; Sang, Y. From the past to the future: Fermented milks and their health effects against human diseases. Food Front. 2023, 4, 1747–1777. https://doi.org/10.1002/fft2.304.
- 32.Miyamoto, M.; Ueno, H.M.; Watanabe, M.; Tatsuma, Y.; Seto, Y.; Miyamoto, T.; Nakajima, H. Distinctive proteolytic activity of cell envelope proteinase of Lactobacillus helveticus isolated from airag, a traditional Mongolian fermented mare's milk. Int. J. Food Microbiol. 2015, 197, 65–71. https://doi.org/10.1016/j.ijfoodmicro.2014.12.012.
- 33.Endo, A.; Irisawa, T.; Dicks, L.; Tanasupawat, S. Fermented Foods | Fermentations of East and Southeast Asia; Elsevier: Amsterdam, The Netherlands, 2014; pp. 846–851. https://doi.org/10.1016/b978-0-12-384730-0.00119-1.
- 34.Anandharaj, M.; Sivasankari, B.; Santhanakaruppu, R.; Manimaran, M.; Rani, R.P.; Sivakumar, S. Determining the probiotic potential of cholesterol-reducing Lactobacillus and Weissella strains isolated from gherkins (fermented cucumber) and south Indian fermented koozh. Res. Microbiol. 2015, 166, 428–439. https://doi.org/10.1016/j.resmic.2015.03.002.
- 35.Nielsen, B.; Gürakan, G.C.; Ünlü, G. Kefir: A Multifaceted Fermented Dairy Product. Probiotics Antimicrob. Proteins 2014, 6, 123–135. https://doi.org/10.1007/s12602-014-9168-0.
- 36.Wu, R.; Wang, L.; Wang, J.; Li, H.; Menghe, B.; Wu, J.; Guo, M.; Zhang, H. Isolation and preliminary probiotic selection of lactobacilli from koumiss in Inner Mongolia. J. Basic Microbiol. 2009, 49, 318–326. https://doi.org/10.1002/jobm.200800047.
- 37.Xiong, T.; Guan, Q.; Song, S.; Hao, M.; Xie, M. Dynamic changes of lactic acid bacteria flora during Chinese sauerkraut fermentation. Food Control 2012, 26, 178–181. https://doi.org/10.1016/j.foodcont.2012.01.027.
- 38.Jung, Y.-J.; Lee, Y.-T.; Ngo, V.L.; Cho, Y.-H.; Ko, E.-J.; Hong, S.-M.; Kim, K.-H.; Jang, J.-H.; Oh, J.-S.; Park, M.-K.; et al. Heat-killed Lactobacillus casei confers broad protection against influenza A virus primary infection and develops heterosubtypic immunity against future secondary infection. Sci. Rep. 2017, 7, 17360 https://doi.org/10.1038/s41598-017-17487-8.
- 39.Xu, H.; Wang, J.; Liu, H.; Shen, G.; Zhang, Z.; Mei, X.; Sun, Y.; Dong, Y.; Wei, D.; Wang, W. Screening of uric acid-degrading lactic acid bacteria consortium and strain combination for enhancing degradation. Microbiol. China 2023, 50, 2545–2555. https://doi.org/10.13344/j.microbiol.china.220830.
- 40.Liu, C.-J.; Tang, X.-D.; Yu, J.; Zhang, H.-Y.; Li, X.-R. Gut microbiota alterations from different Lactobacillus probiotic-fermented yoghurt treatments in slow-transit constipation. J. Funct. Foods 2017, 38, 110–118. https://doi.org/10.1016/j.jff.2017.08.037.
- 41.Anzawa, D.; Mawatari, T.; Tanaka, Y.; Yamamoto, M.; Genda, T.; Takahashi, S.; Nishijima, T.; Kamasaka, H.; Suzuki, S.; Kuriki, T. Effects of synbiotics containing Bifidobacterium animalis subsp. lactis GCL2505 and inulin on intestinal bifidobacteria: A randomized, placebo‐controlled, crossover study. Food Sci. Nutr. 2019, 7, 1828–1837.
- 42.Akhtar, S.; Hussain, M.; Ismail, T.; Riaz, M. Ethnic Fermented Foods of Pakistan; Springer: New Delhi, India, 2016; pp. 119–137. https://doi.org/10.1007/978-81-322-2800-4_5.
- 43.Sakandar, H.A.; Usman, K.; Imran, M. Isolation and characterization of gluten-degrading Enterococcus mundtii and Wickerhamomyces anomalus, potential probiotic strains from indigenously fermented sourdough (Khamir). Lwt 2018, 91, 271–277. https://doi.org/10.1016/j.lwt.2018.01.023.
- 44.Khan, A.; Li, S.; Han, H.; Jin, W.-L.; Ling, Z.; Ji, J.; Iram, S.; Liu, P.; Xiao, S.; Salama, E.-S.; et al. A gluten degrading probiotics relieves CeD symptom and normalize immune system in mice A gluten degrading probiotic Bacillus subtilis LZU-GM relieve adverse effect of gluten additive food and balances gut microbiota in mice. Food Res. Int. 2023, 170, 112960. https://doi.org/10.1016/j.foodres.2023.112960.
- 45.Nahidul-Islam, S.M.; Kuda, T.; Takahashi, H.; Kimura, B. Bacterial and fungal microbiota in traditional Bangladeshi fermented milk products analysed by culture-dependent and culture-independent methods. Food Res. Int. 2018, 111, 431–437. https://doi.org/10.1016/j.foodres.2018.05.048.
- 46.Zhadyra, S.; Han, X.; Anapiyayev, B.B.; Tao, F.; Xu, P. Bacterial diversity analysis in Kazakh fermented milks Shubat and Ayran by combining culture-dependent and culture-independent methods. Lwt 2021, 141, 110877. https://doi.org/10.1016/j.lwt.2021.110877.
- 47.Peres, C.M.; Alves, M.; Hernandez-Mendoza, A.; Moreira, L.; Silva, S.; Bronze, M.R.; Vilas-Boas, L.; Peres, C.; Malcata, F.X. Novel isolates of lactobacilli from fermented Portuguese olive as potential probiotics. LWT Food Sci. Technol. 2014, 59, 234–246. https://doi.org/10.1016/j.lwt.2014.03.003.
- 48.Abriouel, H.; Omar, N.B.; López, R.L.; Martínez-Cañamero, M.; Keleke, S.; Gálvez, A. Culture-independent analysis of the microbial composition of the African traditional fermented foods poto poto and dégué by using three different DNA extraction methods. Int. J. Food Microbiol. 2006, 111, 228–233. https://doi.org/10.1016/j.ijfoodmicro.2006.06.006.
- 49.Liang, T.; Xie, X.; Zhang, J.; Ding, Y.; Wu, Q. Bacterial community and composition of different traditional fermented dairy products in China, South Africa, and Sri Lanka by high-throughput sequencing of 16S rRNA genes. Lwt 2021, 144, 111209. https://doi.org/10.1016/j.lwt.2021.111209.
- 50.Shori, A.B. Camel milk and its fermented products as a source of potential probiotic strains and novel food cultures: A mini review. PharmaNutrition 2017, 5, 84–88. https://doi.org/10.1016/j.phanu.2017.06.003.
- 51.Sengun, I.Y.; Nielsen, D.S.; Karapinar, M.; Jakobsen, M. Identification of lactic acid bacteria isolated from Tarhana, a traditional Turkish fermented food. Int. J. Food Microbiol. 2009, 135, 105–111. https://doi.org/10.1016/j.ijfoodmicro.2009.07.033.
- 52.Das, G.; Heredia, J.B.; de Lourdes Pereira, M.; Coy-Barrera, E.; Rodrigues Oliveira, S.M.; Gutiérrez-Grijalva, E.P.; Cabanillas-Bojórquez, L.A.; Shin, H.-S.; Patra, J.K. Korean traditional foods as antiviral and respiratory disease prevention and treatments: A detailed review. Trends Food Sci. Technol. 2021, 116, 415–433. https://doi.org/10.1016/j.tifs.2021.07.037.
- 53.Patel, A.; Prajapati, J.B.; Holst, O.; Ljungh, A. Determining probiotic potential of exopolysaccharide producing lactic acid bacteria isolated from vegetables and traditional Indian fermented food products. Food Biosci. 2014, 5, 27–33. https://doi.org/10.1016/j.fbio.2013.10.002.
- 54.Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2017, 59, 506–527. https://doi.org/10.1080/10408398.2017.1383355.
- 55.Santiago-López, L.; Aguilar-Toalá, J.E.; Hernández-Mendoza, A.; Vallejo-Cordoba, B.; Liceaga, A.M.; González-Córdova, A.F. Invited review: Bioactive compounds produced during cheese ripening and health effects associated with aged cheese consumption. J. Dairy Sci. 2018, 101, 3742–3757. https://doi.org/10.3168/jds.2017-13465.
- 56.Quigley, L.; O'Sullivan, O.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. Molecular approaches to analysing the microbial composition of raw milk and raw milk cheese. Int. J. Food Microbiol. 2011, 150, 81–94. https://doi.org/10.1016/j.ijfoodmicro.2011.08.001.
- 57.Saithong, P.; Panthavee, W.; Boonyaratanakornkit, M.; Sikkhamondhol, C. Use of a starter culture of lactic acid bacteria in plaa-som, a Thai fermented fish. J. Biosci. Bioeng. 2010, 110, 553–557. https://doi.org/10.1016/j.jbiosc.2010.06.004.
- 58.Barus, T.; Kristani, A.; Yulandi, A.D.I. Diversity of Amylase-Producing Bacillus spp. from “Tape” (Fermented Cassava). HAYATI J. Biosci. 2013, 20, 94–98. https://doi.org/10.4308/hjb.20.2.94.
- 59.Raji, M.N.A.; Ab Karim, S.; Ishak, F.A.C.; Arshad, M.M. Past and present practices of the Malay food heritage and culture in Malaysia. J. Ethn. Foods 2017, 4, 221–231. https://doi.org/10.1016/j.jef.2017.11.001.
- 60.Chettri, R.; Tamang, J.P. Bacillus species isolated from tungrymbai and bekang, naturally fermented soybean foods of India. Int. J. Food Microbiol. 2015, 197, 72–76. https://doi.org/10.1016/j.ijfoodmicro.2014.12.021.
- 61.Mishra, B.K.; Hati, S.; Das, S. Bio-nutritional aspects of Tungrymbai, an ethnic functional fermented soy food of Khasi Hills, Meghalaya, India. Clin. Nutr. Exp. 2019, 26, 8–22. https://doi.org/10.1016/j.yclnex.2019.05.004.
- 62.Dajanta, K.; Apichartsrangkoon, A.; Chukeatirote, E.; Frazier, R.A. Free-amino acid profiles of thua nao, a Thai fermented soybean. Food Chem. 2011, 125, 342–347. https://doi.org/10.1016/j.foodchem.2010.09.002.
- 63.Mahidsanan, T.; Gasaluck, P.; Eumkeb, G. A novel soybean flour as a cryoprotectant in freeze-dried Bacillus subtilis SB-MYP-1. Lwt 2017, 77, 152–159. https://doi.org/10.1016/j.lwt.2016.11.015.
- 64.Cai, H.; Dumba, T.; Sheng, Y.; Li, J.; Lu, Q.; Liu, C.; Cai, C.; Feng, F.; Zhao, M. Microbial diversity and chemical property analyses of sufu products with different producing regions and dressing flavors. Lwt 2021, 144, 111245. https://doi.org/10.1016/j.lwt.2021.111245.
- 65.Yao, D.; Xu, L.; Wu, M.; Wang, X.; Zhu, L.; Wang, C. Effects of microbial community succession on flavor compounds and physicochemical properties during CS sufu fermentation. Lwt 2021, 152, 112313. https://doi.org/10.1016/j.lwt.2021.112313.
- 66.Dank, A.; van Mastrigt, O.; Yang, Z.; Dinesh, V.M.; Lillevang, S.K.; Weij, C.; Smid, E.J. The cross-over fermentation concept and its application in a novel food product: The dairy miso case study. Lwt 2021, 142, 111041. https://doi.org/10.1016/j.lwt.2021.111041.
- 67.Panda, A.; Ghosh, K.; Ray, M.; Nandi, S.K.; Parua, S.; Bera, D.; Singh, S.N.; Dwivedi, S.K.; Mondal, K.C. Ethnic preparation and quality assessment of Chhurpi, a home-made cheese of Ladakh, India. J. Ethn. Foods 2016, 3, 257–262. https://doi.org/10.1016/j.jef.2016.12.004.
- 68.Rai, A.K.; Kumari, R.; Sanjukta, S.; Sahoo, D. Production of bioactive protein hydrolysate using the yeasts isolated from soft chhurpi. Bioresour. Technol. 2016, 219, 239–245. https://doi.org/10.1016/j.biortech.2016.07.129.
- 69.Dewan, S.; Tamang, J.P. Dominant lactic acid bacteria and their technological properties isolated from the Himalayan ethnic fermented milk products. Antonie Van Leeuwenhoek 2007, 92, 343–352. https://doi.org/10.1007/s10482-007-9163-5.
- 70.Todorov, S.D. Diversity of bacteriocinogenic lactic acid bacteria isolated from boza, a cereal-based fermented beverage from Bulgaria. Food Control 2010, 21, 1011–1021. https://doi.org/10.1016/j.foodcont.2009.12.020.
- 71.Yeğin, S.; Üren, A. Biogenic amine content of boza: A traditional cereal-based, fermented Turkish beverage. Food Chem. 2008, 111, 983–987. https://doi.org/10.1016/j.foodchem.2008.05.020.
- 72.Chao, S.-H.; Wu, R.-J.; Watanabe, K.; Tsai, Y.-C. Diversity of lactic acid bacteria in suan-tsai and fu-tsai, traditional fermented mustard products of Taiwan. Int. J. Food Microbiol. 2009, 135, 203–210. https://doi.org/10.1016/j.ijfoodmicro.2009.07.032.
- 73.Nguyen, D.T.L.; Van Hoorde, K.; Cnockaert, M.; De Brandt, E.; De Bruyne, K.; Le, B.T.; Vandamme, P. A culture-dependent and -independent approach for the identification of lactic acid bacteria associated with the production of nem chua, a Vietnamese fermented meat product. Food Res. Int. 2013, 50, 232–240. https://doi.org/10.1016/j.foodres.2012.09.029.
- 74.Tomita, S.; Watanabe, J.; Kuribayashi, T.; Tanaka, S.; Kawahara, T. Metabolomic evaluation of different starter culture effects on water-soluble and volatile compound profiles in nozawana pickle fermentation. Food Chem. Mol. Sci. 2021, 2, 100019. https://doi.org/10.1016/j.fochms.2021.100019.
- 75.Daliri, E.B.-M.; Tyagi, A.; Ofosu, F.K.; Chelliah, R.; Kim, J.-H.; Kim, J.-R.; Yoo, D.; Oh, D.-H. A discovery-based metabolomic approach using UHPLC Q-TOF MS/MS unveils a plethora of prospective antihypertensive compounds in Korean fermented soybeans. Lwt 2021, 137, 110399. https://doi.org/10.1016/j.lwt.2020.110399.
- 76.Zha, M.; Li, K.; Zhang, W.; Sun, Z.; Kwok, L.-Y.; Menghe, B.; Chen, Y. Untargeted mass spectrometry-based metabolomics approach unveils molecular changes in milk fermented by Lactobacillus plantarum P9. Lwt 2021, 140, 110759. https://doi.org/10.1016/j.lwt.2020.110759.
- 77.Muhialdin, B.J.; Zawawi, N.; Abdull Razis, A.F.; Bakar, J.; Zarei, M. Antiviral activity of fermented foods and their probiotics bacteria towards respiratory and alimentary tracts viruses. Food Control 2021, 127, 108140. https://doi.org/10.1016/j.foodcont.2021.108140.
- 78.Chan, M.Z.A.; Lau, H.; Lim, S.Y.; Li, S.F.Y.; Liu, S.-Q. Untargeted LC-QTOF-MS/MS based metabolomics approach for revealing bioactive components in probiotic fermented coffee brews. Food Res. Int. 2021, 149, 110656. https://doi.org/10.1016/j.foodres.2021.110656.
- 79.Ayyash, M.; Abdalla, A.; Alhammadi, A.; Senaka Ranadheera, C.; Affan Baig, M.; Al-Ramadi, B.; Chen, G.; Kamal-Eldin, A.; Huppertz, T. Probiotic survival, biological functionality and untargeted metabolomics of the bioaccessible compounds in fermented camel and bovine milk after in vitro digestion. Food Chem. 2021, 363, 130243. https://doi.org/10.1016/j.foodchem.2021.130243.
- 80.Jia, W.; Liu, Y.; Shi, L. Integrated metabolomics and lipidomics profiling reveals beneficial changes in sensory quality of brown fermented goat milk. Food Chem. 2021, 364, 130378. https://doi.org/10.1016/j.foodchem.2021.130378.
- 81.Kang, H.J.; Yang, H.J.; Kim, M.J.; Han, E.-S.; Kim, H.-J.; Kwon, D.Y. Metabolomic analysis of meju during fermentation by ultra performance liquid chromatography-quadrupole-time of flight mass spectrometry (UPLC-Q-TOF MS). Food Chem. 2011, 127, 1056–1064. https://doi.org/10.1016/j.foodchem.2011.01.080.
- 82.Ferri, M.; Serrazanetti, D.I.; Tassoni, A.; Baldissarri, M.; Gianotti, A. Improving the functional and sensorial profile of cereal-based fermented foods by selecting Lactobacillus plantarum strains via a metabolomics approach. Food Res. Int. 2016, 89, 1095–1105. https://doi.org/10.1016/j.foodres.2016.08.044.
- 83.Gao, Y.X.; Xu, B.; Fan, H.R.; Zhang, M.R.; Zhang, L.J.; Lu, C.; Zhang, N.N.; Fan, B.; Wang, F.Z.; Li, S. 1H NMR-based chemometric metabolomics characterization of soymilk fermented by Bacillus subtilis BSNK-5. Food Res. Int. 2020, 138, 109686. https://doi.org/10.1016/j.foodres.2020.109686.
- 84.Lee, D.E.; Shin, G.R.; Lee, S.; Jang, E.S.; Shin, H.W.; Moon, B.S.; Lee, C.H. Metabolomics reveal that amino acids are the main contributors to antioxidant activity in wheat and rice gochujangs (Korean fermented red pepper paste). Food Res. Int. 2016, 87, 10–17. https://doi.org/10.1016/j.foodres.2016.06.015.
- 85.García-García, A.B.; Lamichhane, S.; Castejón, D.; Cambero, M.I.; Bertram, H.C. 1H HR-MAS NMR-based metabolomics analysis for dry-fermented sausage characterization. Food Chem. 2018, 240, 514–523. https://doi.org/10.1016/j.foodchem.2017.07.150.
- 86.Tomita, S.; Nakamura, T.; Okada, S. NMR- and GC/MS-based metabolomic characterization of sunki , an unsalted fermented pickle of turnip leaves. Food Chem. 2018, 258, 25–34. https://doi.org/10.1016/j.foodchem.2018.03.038.
- 87.Xia, Y.; Yu, J.; Miao, W.; Shuang, Q. A UPLC-Q-TOF-MS-based metabolomics approach for the evaluation of fermented mare’s milk to koumiss. Food Chem. 2020, 320, 126619. https://doi.org/10.1016/j.foodchem.2020.126619.
- 88.Qu, Q.; Yang, F.; Zhao, C.; Liu, X.; Yang, P.; Li, Z.; Han, L.; Shi, X. Effects of fermented ginseng on the gut microbiota and immunity of rats with antibiotic-associated diarrhea. J. Ethnopharmacol. 2021, 267, 113594. https://doi.org/10.1016/j.jep.2020.113594.
- 89.Ghyselinck, J.; Verstrepen, L.; Moens, F.; Van Den Abbeele, P.; Bruggeman, A.; Said, J.; Smith, B.; Barker, L.A.; Jordan, C.; Leta, V.; et al. Influence of probiotic bacteria on gut microbiota composition and gut wall function in an in-vitro model in patients with Parkinson's disease. Int. J. Pharm. X 2021, 3, 100087. https://doi.org/10.1016/j.ijpx.2021.100087.
- 90.Banwo, K.; Olojede, A.O.; Adesulu-Dahunsi, A.T.; Verma, D.K.; Thakur, M.; Tripathy, S.; Singh, S.; Patel, A.R.; Gupta, A.K.; Aguilar, C.N.; et al. Functional importance of bioactive compounds of foods with Potential Health Benefits: A review on recent trends. Food Biosci. 2021, 43, 101320. https://doi.org/10.1016/j.fbio.2021.101320.
- 91.Jabbari, F.; Babaeipour, V.; Saharkhiz, S. Comprehensive review on biosynthesis of hyaluronic acid with different molecular weights and its biomedical applications. Int. J. Biol. Macromol. 2023, 240, 124484. https://doi.org/10.1016/j.ijbiomac.2023.124484.
- 92.Wan, Y.-J.; Hong, T.; Shi, H.-F.; Yin, J.-Y.; Koev, T.; Nie, S.-P.; Gilbert, R.G.; Xie, M.-Y. Probiotic fermentation modifies the structures of pectic polysaccharides from carrot pulp. Carbohydr. Polym. 2021, 251, 117116. https://doi.org/10.1016/j.carbpol.2020.117116.
- 93.Zhang, Y.; Zhang, J.; Yan, J.; Qi, X.; Wang, Y.; Zheng, Z.; Liang, J.; Ling, J.; Chen, Y.; Tang, X.; et al. Application of fermented Chinese herbal medicines in food and medicine field: From an antioxidant perspective. Trends Food Sci. Technol. 2024, 148, 104410. https://doi.org/10.1016/j.tifs.2024.104410.
- 94.Lee, K.S.; Park, S.N. Cytoprotective effects and mechanisms of quercetin, quercitrin and avicularin isolated from Lespedeza cuneata G. Don against ROS-induced cellular damage. J. Ind. Eng. Chem. 2019, 71, 160–166. https://doi.org/10.1016/j.jiec.2018.11.018.
- 95.Huang, F.; Hong, R.; Zhang, R.; Yi, Y.; Dong, L.; Liu, L.; Jia, X.; Ma, Y.; Zhang, M. Physicochemical and biological properties of longan pulp polysaccharides modified by Lactobacillus fermentum fermentation. Int. J. Biol. Macromol. 2019, 125, 232–237. https://doi.org/10.1016/j.ijbiomac.2018.12.061.
- 96.Fernandez-Julia, P.J.; Munoz-Munoz, J.; van Sinderen, D. A comprehensive review on the impact of β-glucan metabolism by Bacteroides and Bifidobacterium species as members of the gut microbiota. Int. J. Biol. Macromol. 2021, 181, 877–889. https://doi.org/10.1016/j.ijbiomac.2021.04.069.
- 97.Hu, T.; Shi, S.; Ma, Q. Modulation effects of microorganisms on tea in fermentation. Front. Nutr. 2022, 9, 931790. https://doi.org/10.3389/fnut.2022.931790.
- 98.Guo, W.; Chen, M.; Cui, S.; Tang, X.; Zhang, Q.; Zhao, J.; Mao, B.; Zhang, H. Effects of Lacticaseibacillus casei fermentation on the bioactive compounds, volatile and non-volatile compounds, and physiological properties of barley beverage. Food Biosci. 2023, 53, 102695. https://doi.org/10.1016/j.fbio.2023.102695.
- 99.Choi, Y.; Bose, S.; Shin, N.R.; Song, E.-J.; Nam, Y.-D.; Kim, H. Lactate-Fortified Puerariae Radix Fermented by Bifidobacterium breve Improved Diet-Induced Metabolic Dysregulation via Alteration of Gut Microbial Communities. Nutrients 2020, 12, 276. https://doi.org/10.3390/nu12020276.
- 100.Li, L.; Wang, L.; Fan, W.; Jiang, Y.; Zhang, C.; Li, J.; Peng, W.; Wu, C. The Application of Fermentation Technology in Traditional Chinese Medicine: A Review. Am. J. Chin. Med. 2020, 48, 899–921. https://doi.org/10.1142/s0192415x20500433.
- 101.Seong, J.S.; Xuan, S.H.; Park, S.H.; Lee, K.S.; Park, Y.M.; Park, S.N. Antioxidative and Antiaging Activities and Component Analysis of Lespedeza cuneata G. Don Extracts Fermented with Lactobacillus pentosus. J. Microbiol. Biotechnol. 2017, 27, 1961–1970. https://doi.org/10.4014/jmb.1706.06028.
- 102.Li, C.; Ding, Q.; Nie, S.-P.; Zhang, Y.-S.; Xiong, T.; Xie, M.-Y. Carrot Juice Fermented with Lactobacillus plantarum NCU116 Ameliorates Type 2 Diabetes in Rats. J. Agric. Food Chem. 2014, 62, 11884–11891. https://doi.org/10.1021/jf503681r.
- 103.Song, S.; Liu, X.; Zhao, B.; Abubaker, M.A.; Huang, Y.; Zhang, J. Effects of Lactobacillus plantarum Fermentation on the Chemical Structure and Antioxidant Activity of Polysaccharides from Bulbs of Lanzhou Lily. ACS Omega 2021, 6, 29839–29851. https://doi.org/10.1021/acsomega.1c04339.
- 104.Wang, R.; Sun, J.; Lassabliere, B.; Yu, B.; Liu, S.Q. Green tea fermentation with Saccharomyces boulardii CNCM I-745 and Lactiplantibacillus plantarum 299V. Lwt 2022, 157, 113081. https://doi.org/10.1016/j.lwt.2022.113081.
- 105.Zhang, D.-N.; Guo, X.-Y.; Chen, Z.-G. A novel and efficient method for the isolation and purification of polysaccharides from lily bulbs by Saccharomyces cerevisiae fermentation. Process Biochem. 2014, 49, 2299–2304. https://doi.org/10.1016/j.procbio.2014.09.004.
- 106.Kuo-ChingWen; Lin, S.-P.; Yu, C.-P.; Chiang, H.-M. Comparison of Puerariae Radix and Its Hydrolysate on Stimulation of Hyaluronic Acid Production in NHEK Cells. Am. J. Chin. Med. 2010, 38, 143–155.
- 107.Yang, H.J.; Kwon, D.Y.; Moon, N.R.; Kim, M.J.; Kang, H.J.; Jung, D.Y.; Park, S. Soybean fermentation with Bacillus licheniformis increases insulin sensitizing and insulinotropic activity. Food Funct. 2013, 4, 1675. https://doi.org/10.1039/c3fo60198f.
- 108.Okamoto, T.; Sugimoto, S.; Noda, M.; Yokooji, T.; Danshiitsoodol, N.; Higashikawa, F.; Sugiyama, M. Interleukin-8 Release Inhibitors Generated by Fermentation of Artemisia princeps Pampanini Herb Extract With Lactobacillus plantarum SN13T. Front. Microbiol. 2020, 11, 1159. https://doi.org/10.3389/fmicb.2020.01159.
- 109.Lin, Y.-W.; Mou, Y.-C.; Su, C.-C.; Chiang, B.-H. Antihepatocarcinoma Activity of Lactic Acid Bacteria Fermented Panax notoginseng. J. Agric. Food Chem. 2010, 58, 8528–8534. https://doi.org/10.1021/jf101543k.
- 110.Jin, S.; Luo, M.; Wang, W.; Zhao, C.-j.; Gu, C.-b.; Li, C.-y.; Zu, Y.-g.; Fu, Y.-j.; Guan, Y. Biotransformation of polydatin to resveratrol in Polygonum cuspidatum roots by highly immobilized edible Aspergillus niger and Yeast. Bioresour. Technol. 2013, 136, 766–770. https://doi.org/10.1016/j.biortech.2013.03.027.
- 111.Sheih, I.C.; Fang, T.J.; Wu, T.-K.; Chang, C.-H.; Chen, R.-Y. Purification and Properties of a Novel Phenolic Antioxidant from Radix astragali Fermented by Aspergillus oryzae M29. J. Agric. Food Chem 2011, 59, 6520–6525. https://doi.org/10.1021/jf2011547.
- 112.Kwon, H.-K.; Jo, W.-R.; Park, H.-J. Immune-enhancing activity of C. militaris fermented with Pediococcus pentosaceus (GRC-ON89A) in CY-induced immunosuppressed model. BMC Complement. Altern. Med. 2018, 18, 75. https://doi.org/10.1186/s12906-018-2133-9.
- 113.Pyo, Y.; Kwon, K.H.; Jung, Y.J. Probiotic Functions in Fermented Foods: Anti-Viral, Immunomodulatory, and Anti-Cancer Benefits. Foods 2024, 13, 2386. https://doi.org/10.3390/foods13152386.
- 114.Li, K.; Park, M.-K.; Ngo, V.; Kwon, Y.-M.; Lee, Y.-T.; Yoo, S.; Cho, Y.-H.; Hong, S.-M.; Hwang, H.S.; Ko, E.-J.; et al. Lactobacillus plantarum DK119 as a Probiotic Confers Protection against Influenza Virus by Modulating Innate Immunity. PLoS ONE 2013, 8, e0075368. https://doi.org/10.1371/journal.pone.0075368.
- 115.Makino, S.; Sato, A.; Goto, A.; Nakamura, M.; Ogawa, M.; Chiba, Y.; Hemmi, J.; Kano, H.; Takeda, K.; Okumura, K.; et al. Enhanced natural killer cell activation by exopolysaccharides derived from yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. J. Dairy Sci. 2016, 99, 915–923. https://doi.org/10.3168/jds.2015-10376.
- 116.Burrell, C.J.; Howard, C.R.; Murphy, F.A. Pathogenesis of Virus Infections. In Fenner and White's Medical Virology; Academic Press: Cambridge, MA, USA, 2017; pp. 77–104.
- 117.Zhao, W.; Wang, L.; Liu, M.; Zhang, D.; Andika, I.B.; Zhu, Y.; Sun, L. A Reduced Starch Level in Plants at Early Stages of Infection by Viruses Can Be Considered a Broad-Range Indicator of Virus Presence. Viruses 2022, 14, 1176. https://doi.org/10.3390/v14061176.
- 118.Zhu, S.; Viejo-Borbolla, A. Pathogenesis and virulence of herpes simplex virus. Virulence 2021, 12, 2670–2702. https://doi.org/10.1080/21505594.2021.1982373.
- 119.Michels, M.; Jesus, G.F.A.; Abatti, M.R.; Córneo, E.; Cucker, L.; de Medeiros Borges, H.; da Silva Matos, N.; Rocha, L.B.; Dias, R.; Simon, C.S.; et al. Effects of different probiotic strains B. lactis, L. rhamnosus and L. reuteri on brain-intestinal axis immunomodulation in an endotoxin-induced inflammation. Mol. Neurobiol. 2022, 59, 5168–5178. https://doi.org/10.1007/s12035-022-02906-3.
- 120.Mousavi, E.; Makvandi, M.; Teimoori, A.; Ataei, A.; Ghafari, S.; Samarbaf-Zadeh, A. Antiviral effects of Lactobacillus crispatus against HSV-2 in mammalian cell lines. J. Chin. Med. Assoc. 2018, 81, 262–267. https://doi.org/10.1016/j.jcma.2017.07.010.
- 121.Filip, R.; Gheorghita Puscaselu, R.; Anchidin-Norocel, L.; Dimian, M.; Savage, W.K. Global Challenges to Public Health Care Systems during the COVID-19 Pandemic: A Review of Pandemic Measures and Problems. J. Pers. Med. 2022, 12, 1295. https://doi.org/10.3390/jpm12081295.
- 122.Li, D.; Breiman, A.; le Pendu, J.; Uyttendaele, M. Anti-viral Effect of Bifidobacterium adolescentis against Noroviruses. Front. Microbiol. 2016, 7, 864. https://doi.org/10.3389/fmicb.2016.00864.
- 123.Choi, H.-J.; Song, J.-H.; Ahn, Y.-J.; Baek, S.-H.; Kwon, D.-H. Antiviral activities of cell-free supernatants of yogurts metabolites against some RNA viruses. Eur. Food Res. Technol. 2009, 228, 945–950. https://doi.org/10.1007/s00217-009-1009-0.
- 124.Santos, A.; San Mauro, M.; Sanchez, A.; Torres, J.M.; Marquina, D. The Antimicrobial Properties of Different Strains of Lactobacillus spp. Isolated from Kefir. Syst. Appl. Microbiol. 2003, 26, 434–437. https://doi.org/10.1078/072320203322497464.
- 125.Shojadoost, B.; Kulkarni, R.R.; Brisbin, J.T.; Quinteiro-Filho, W.; Alkie, T.N.; Sharif, S. Interactions between lactobacilli and chicken macrophages induce antiviral responses against avian influenza virus. Res. Vet. Sci. 2019, 125, 441–450. https://doi.org/10.1016/j.rvsc.2017.10.007.
- 126.Ermolenko, E.I.; Desheva, Y.A.; Kolobov, A.A.; Kotyleva, M.P.; Sychev, I.A.; Suvorov, A.N. Anti–Influenza Activity of Enterocin B In vitro and Protective Effect of Bacteriocinogenic Enterococcal Probiotic Strain on Influenza Infection in Mouse Model. Probiotics Antimicrob. Proteins 2018, 11, 705–712. https://doi.org/10.1007/s12602-018-9457-0.
- 127.Nalbantoglu, U.; Cakar, A.; Dogan, H.; Abaci, N.; Ustek, D.; Sayood, K.; Can, H. Metagenomic analysis of the microbial community in kefir grains. Food Microbiol. 2014, 41, 42–51. https://doi.org/10.1016/j.fm.2014.01.014.
- 128.Ang, L.Y.E.; Too, H.K.I.; Tan, E.L.; Chow, T.-K.V.; Shek, P.-C.L.; Tham, E.; Alonso, S. Antiviral activity of Lactobacillus reuteri Protectis against Coxsackievirus A and Enterovirus 71 infection in human skeletal muscle and colon cell lines. Virol. J. 2016, 13, 111. https://doi.org/10.1186/s12985-016-0567-6.
- 129.Kawashima, T.; Hayashi, K.; Kosaka, A.; Kawashima, M.; Igarashi, T.; Tsutsui, H.; Tsuji, N.M.; Nishimura, I.; Hayashi, T.; Obata, A. Lactobacillus plantarum strain YU from fermented foods activates Th1 and protective immune responses. Int. Immunopharmacol. 2011, 11, 2017–2024. https://doi.org/10.1016/j.intimp.2011.08.013.
- 130.Li, K.; Park, M.-K.; Ngo, V.; Kwon, Y.-M.; Lee, Y.-T.; Yoo, S.; Cho, Y.-H.; Hong, S.-M.; Hwang, H.S.; Ko, E.-J.; et al. Lactobacillus plantarum DK119 as a Probiotic Confers Protection against Influenza Virus by Modulating Innate Immunity. PLoS ONE 2013, 8, e75368. https://doi.org/10.1371/journal.pone.0075368.
- 131.Nakayama, Y.; Moriya, T.; Sakai, F.; Ikeda, N.; Shiozaki, T.; Hosoya, T.; Nakagawa, H.; Miyazaki, T. Oral administration of Lactobacillus gasseri SBT2055 is effective for preventing influenza in mice. Sci. Rep. 2014, 4, 4638. https://doi.org/10.1038/srep04638.
- 132.Botic, T.; Klingberg, T.; Weingartl, H.; Cencic, A. A novel eukaryotic cell culture model to study antiviral activity of potential probiotic bacteria. Int. J. Food Microbiol. 2007, 115, 227–234. https://doi.org/10.1016/j.ijfoodmicro.2006.10.044.
- 133.Ivec, M.; Botić, T.; Koren, S.; Jakobsen, M.; Weingartl, H.; Cencič, A. Interactions of macrophages with probiotic bacteria lead to increased antiviral response against vesicular stomatitis virus. Antivir. Res. 2007, 75, 266–274. https://doi.org/10.1016/j.antiviral.2007.03.013.
- 134.Grant, W.; Lahore, H.; McDonnell, S.; Baggerly, C.; French, C.; Aliano, J.; Bhattoa, H. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. https://doi.org/10.3390/nu12040988.
- 135.Calder, P.; Carr, A.; Gombart, A.; Eggersdorfer, M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients 2020, 12, 1181. https://doi.org/10.3390/nu12041181.
- 136.Wu, G. Important roles of dietary taurine, creatine, carnosine, anserine and 4-hydroxyproline in human nutrition and health. Amino Acids 2020, 52, 329–360. https://doi.org/10.1007/s00726-020-02823-6.
- 137.Chen, Z.; Liang, W.; Liang, J.; Dou, J.; Guo, F.; Zhang, D.; Xu, Z.; Wang, T. Probiotics: Functional food ingredients with the potential to reduce hypertension. Front. Cell. Infect. Microbiol. 2023, 13, 1220877. https://doi.org/10.3389/fcimb.2023.1220877.
- 138.Voidarou, C.; Antoniadou, Μ.; Rozos, G.; Tzora, A.; Skoufos, I.; Varzakas, T.; Lagiou, A.; Bezirtzoglou, E. Fermentative Foods: Microbiology, Biochemistry, Potential Human Health Benefits and Public Health Issues. Foods 2020, 10, 69. https://doi.org/10.3390/foods10010069.
How to Cite
Zhou, X.; Cui, B.; Wang, X.; Khan, A.; Wang, W. Fermented Foods Strengthen Immunity against Viral Infections. Health and Metabolism 2025, 2 (4), 3. https://doi.org/10.53941/hm.2025.100026.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


