For many years, excessive intake of SFAs has been recognized as a principal cause of cardiovascular disease (CVD). Despite the prevalence of guidelines recommending the restriction of SFAs intake, particularly among patients with atherosclerotic cardiovascular disease and dyslipidemia, some researchers have pointed out a new connection between SFAs intake and cardiovascular health. This review explores the sources of SFAs in humans and the controversy surrounding the link between different chain lengths of SFAs and CVD risk by analyzing the available evidence, focusing on the effects of SFAs carbon chain heterogeneity. Based on the latest mechanistic studies and epidemiological data, it offers evidence-informed recommendations for clinical practice and dietary guideline amendments. In the authors’ view, the cardiovascular effects of SFAs depend not only on chain length but also on the food matrix in which they are consumed and the nutrients they displace.
- Open Access
- Review
Diverse Mechanisms of Saturated Fatty Acids in Cardiovascular Disease
- Xiaoshan Huang 1, 2,
- Dawit Adisu Tadese 1, 2,
- Qiumin Lu 1,
- Ren Lai 1, *
Author Information
Received: 26 Jun 2025 | Revised: 22 Jul 2025 | Accepted: 14 Aug 2025 | Published: 23 Sep 2025
Abstract
Graphical Abstract
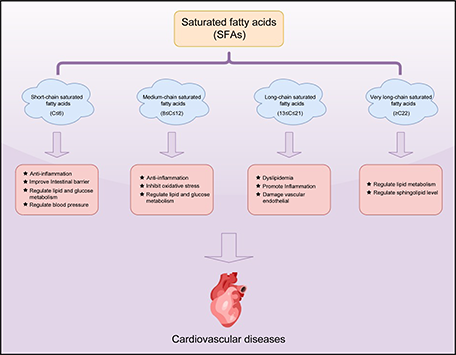
Keywords
References
- 1.Rabadia, J.P.; Thite, V.S.; Desai, B.K.; Bera, R.G.; Patel, S. Cardiovascular System, Its Functions and Disorders. In Cardioprotective Plants; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–34.
- 2.Thiriet, M. Cardiovascular disease: An introduction. In Vasculopathies: Behavioral, Chemical, Environmental, and Genetic Factors; Springer: Cham, Switzerland, 2018.
- 3.Gadó, K.; Szabo, A.; Markovics, D.; Virág, A. Most common cardiovascular diseases of the elderly–A review article. Dev. Health Sci. 2022, 4, 27–32.
- 4.Celermajer, D.S.; Chow, C.K.; Marijon, E.; Anstey, N.M.; Woo, K.S. Cardiovascular disease in the developing world: Prevalences, patterns, and the potential of early disease detection. J. Am. Coll. Cardiol. 2012, 60, 1207–1216.
- 5.Gaziano, T.A. Cardiovascular diseases worldwide. Public Health Approach Cardiovasc. Dis. Prev. Manag 2022, 1, 8–18.
- 6.Countdown, N. NCD Countdown 2030: Worldwide trends in non-communicable disease mortality and progress towards Sustainable Development Goal target 3.4. Lancet 2018, 392, 1072–1088.
- 7.Joseph, P.; Leong, D.; McKee, M.; Anand, S.S.; Schwalm, J.-D.; Teo, K.; Mente, A.; Yusuf, S. Reducing the global burden of cardiovascular disease, part 1: The epidemiology and risk factors. Circ. Res. 2017, 121, 677–694.
- 8.Ferreri, C.; Masi, A.; Sansone, A.; Giacometti, G.; Larocca, A.V.; Menounou, G.; Scanferlato, R.; Tortorella, S.; Rota, D.; Conti, M. Fatty acids in membranes as homeostatic, metabolic and nutritional biomarkers: Recent advancements in analytics and diagnostics. Diagnostics 2016, 7, 1.
- 9.Harika, R.K.; Eilander, A.; Alssema, M.; Osendarp, S.J.; Zock, P.L. Intake of fatty acids in general populations worldwide does not meet dietary recommendations to prevent coronary heart disease: A systematic review of data from 40 countries. Ann. Nutr. Metab. 2013, 63, 229–238.
- 10.Hodson, L.; Skeaff, C.M.; Fielding, B.A. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog. Lipid Res. 2008, 47, 348–380.
- 11.Nicolosi, R.J.; Wilson, T.A.; Lawton, C.; Handelman, G.J. Dietary effects on cardiovascular disease risk factors: Beyond saturated fatty acids and cholesterol. J. Am. Coll. Nutr. 2001, 20, 421S–427S.
- 12.Ackman, R.G. Fatty acids. Mar. Biog. Lipids Fats Oils 1989, 1, 103–137.
- 13.Ratnayake, W.N.; Galli, C. Fat and fatty acid terminology, methods of analysis and fat digestion and metabolism. Ann. Nutr. Metab. 2009, 55, 8–43.
- 14.Rustan, A.C.; Drevon, C.A. Fatty acids: Structures and properties. Encycl. Life Sci. 2005, 1, 1–7.
- 15.Agregán, R.; Popova, T.; López-Pedrouso, M.; Cantalapiedra, J.; Lorenzo, J.M.; Franco, D. Fatty acids. In Food Lipids; Elsevier: 2022; pp. 257–286.
- 16.Maltsev, Y.; Maltseva, K. Fatty acids of microalgae: Diversity and applications. Rev. Environ. Sci. Bio/Technol. 2021, 20, 515–547.
- 17.Markowiak-Kopeć, P.; Śliżewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020, 12, 1107.
- 18.Pouteau, E.; Nguyen, P.; Ballèvre, O.; Krempf, M. Production rates and metabolism of short-chain fatty acids in the colon and whole body using stable isotopes. Proc. Nutr. Soc. 2003, 62, 87–93.
- 19.Macfarlane, S.; Macfarlane, G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003, 62, 67–72.
- 20.Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345.
- 21.Snyder, N.W.; Basu, S.S.; Worth, A.J.; Mesaros, C.; Blair, I.A. Metabolism of propionic acid to a novel acyl-coenzyme A thioester by mammalian cell lines and platelets. J. Lipid Res. 2015, 56, 142–150.
- 22.Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41.
- 23.Trachsel, J.; Bayles, D.O.; Looft, T.; Levine, U.Y.; Allen, H.K. Function and phylogeny of bacterial butyryl coenzyme a: Acetate transferases and their diversity in the proximal colon of swine. Appl. Environ. Microbiol. 2016, 82, 6788–6798.
- 24.Adeva-Andany, M.M.; Carneiro-Freire, N.; Seco-Filgueira, M.; Fernández-Fernández, C.; Mouriño-Bayolo, D. Mitochondrial β-oxidation of saturated fatty acids in humans. Mitochondrion 2019, 46, 73–90.
- 25.Wong, J.M.; De Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243.
- 26.Khatibjoo, A.; Mahmoodi, M.; Fattahnia, F.; Akbari-Gharaei, M.; Shokri, A.-N.; Soltani, S. Effects of dietary short-and medium-chain fatty acids on performance, carcass traits, jejunum morphology, and serum parameters of broiler chickens. J. Appl. Anim. Res. 2018, 46, 492–498.
- 27.Tvrzicka, E.; Kremmyda, L.-S.; Stankova, B.; Zak, A. Fatty acids as biocompounds: Their role in human metabolism, health and disease-a review. Part 1: Classification, dietary sources and biological functions. Biomed. Pap. Med. Fac. Palacky Univ. Olomouc 2011, 155, 117–130.
- 28.Lindmark Månsson, H. Fatty acids in bovine milk fat. Food Nutr. Res. 2008, 52, 1821.
- 29.Babayan, V.K. Medium chain triglycerides and structured lipids. Lipids 1987, 22, 417–420.
- 30.Uchida, Y. The role of fatty acid elongation in epidermal structure and function. Derm.-Endocrinol. 2011, 3, 65–69.
- 31.Shi, H.; Wu, M.; Zhu, J.; Zhang, C.; Yao, D.; Luo, J.; Loor, J. Fatty acid elongase 6 plays a role in the synthesis of long-chain fatty acids in goat mammary epithelial cells. J. Dairy Sci. 2017, 100, 4987–4995.
- 32.Clemente, J.C.; Manasson, J.; Scher, J.U. The role of the gut microbiome in systemic inflammatory disease. BMJ 2018, 360, j5145.
- 33.Zhu, Z.; Hu, Y.; Teixeira, P.G.; Pereira, R.; Chen, Y.; Siewers, V.; Nielsen, J. Multidimensional engineering of Saccharomyces cerevisiae for efficient synthesis of medium-chain fatty acids. Nat. Catal. 2020, 3, 64–74.
- 34.Green, P.R.; Kemper, J.; Schechtman, L.; Guo, L.; Satkowski, M.; Fiedler, S.; Steinbüchel, A.; Rehm, B.H. Formation of short chain length/medium chain length polyhydroxyalkanoate copolymers by fatty acid β-oxidation inhibited Ralstonia eutropha. Biomacromolecules 2002, 3, 208–213.
- 35.Sousa, D.Z.; Smidt, H.; Alves, M.M.; Stams, A.J. Ecophysiology of syntrophic communities that degrade saturated and unsaturated long-chain fatty acids. FEMS Microbiol. Ecol. 2009, 68, 257–272.
- 36.Hillgartner, F.B.; Salati, L.M.; Goodridge, A.G. Physiological and molecular mechanisms involved in nutritional regulation of fatty acid synthesis. Physiol. Rev. 1995, 75, 47–76.
- 37.Günenc, A.N.; Graf, B.; Stark, H.; Chari, A. Fatty acid synthase: Structure, function, and regulation. In Macromolecular Protein Complexes IV; Springer: Berlin/Heidelberg, Germany, 2022.
- 38.Jakobsson, A.; Westerberg, R.; Jacobsson, A. Fatty acid elongases in mammals: Their regulation and roles in metabolism. Prog. Lipid Res. 2006, 45, 237–249.
- 39.Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.-J. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388.
- 40.Baltić, B.; Starčević, M.; Đorđević, J.; Mrdović, B.; Marković, R. Importance of medium chain fatty acids in animal nutrition. In Proceedings of the IOP Conference Series: Earth and Environmental Science, 2017; p. 012048.
- 41.Phuah, E.-T.; Yap, J.W.-L.; Lau, C.-W.; Lee, Y.-Y.; Tang, T.-K. Vegetable oils and animal fats: Sources, properties and recovery. In Recent Advances in Edible Fats and Oils Technology: Processing, Health Implications, Economic and Environmental Impact; Springer: Berlin/Heidelberg, Germany, 2022.
- 42.Nagpal, T.; Sahu, J.K.; Khare, S.K.; Bashir, K.; Jan, K. Trans fatty acids in food: A review on dietary intake, health impact, regulations and alternatives. J. Food Sci. 2021, 86, 5159–5174.
- 43.Takeuchi, T.; Kameyama, K.; Miyauchi, E.; Nakanishi, Y.; Kanaya, T.; Fujii, T.; Kato, T.; Sasaki, T.; Tachibana, N.; Negishi, H. Fatty acid overproduction by gut commensal microbiota exacerbates obesity. Cell Metab. 2023, 35, 361–375. e369.
- 44.Zhao, L.; Huang, Y.; Lu, L.; Yang, W.; Huang, T.; Lin, Z.; Lin, C.; Kwan, H.; Wong, H.L.X.; Chen, Y. Saturated long-chain fatty acid-producing bacteria contribute to enhanced colonic motility in rats. Microbiome 2018, 6, 1–16.
- 45.Poulos, A. Very long chain fatty acids in higher animals—A review. Lipids 1995, 30, 1–14.
- 46.Choi, J.-K. Physical and Biochemical Studies of Saturated Very Long Chain Fatty Acids; Boston University: Boston, MA, USA, 2001.
- 47.Ohno, Y.; Suto, S.; Yamanaka, M.; Mizutani, Y.; Mitsutake, S.; Igarashi, Y.; Sassa, T.; Kihara, A. ELOVL1 production of C24 acyl-CoAs is linked to C24 sphingolipid synthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 18439–18444.
- 48.Guillou, H.; Zadravec, D.; Martin, P.G.; Jacobsson, A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. Prog. Lipid Res. 2010, 49, 186–199.
- 49.Tietel, Z.; Hammann, S.; Meckelmann, S.W.; Ziv, C.; Pauling, J.K.; Wölk, M.; Würf, V.; Alves, E.; Neves, B.; Domingues, M.R. An overview of food lipids toward food lipidomics. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4302–4354.
- 50.Deepu, V.; Rai, V.; Agrawal, D.K. Interaction Between Genetic and Environmental Factors in the Pathogenesis of Cardiovascular Disease. In Environmental Factors in the Pathogenesis of Cardiovascular Diseases; Springer: Berlin/Heidelberg, Germany, 2024; pp. 351–382.
- 51.Ahmad, F.S.; Ning, H.; Rich, J.D.; Yancy, C.W.; Lloyd-Jones, D.M.; Wilkins, J.T. Hypertension, obesity, diabetes, and heart failure–free survival: The cardiovascular disease lifetime risk pooling project. JACC Heart Fail. 2016, 4, 911–919.
- 52.Teo, K.K.; Rafiq, T. Cardiovascular risk factors and prevention: A perspective from developing countries. Can. J. Cardiol. 2021, 37, 733–743.
- 53.Mozaffarian, D.; Wilson, P.W.; Kannel, W.B. Beyond established and novel risk factors: Lifestyle risk factors for cardiovascular disease. Circulation 2008, 117, 3031–3038.
- 54.Stanner, S.; Coe, S.; Frayn, K.N. Cardiovascular Disease: Diet, Nutrition and Emerging Risk Factors; John Wiley & Sons: Hoboken, NJ, USA, 2019.
- 55.Haskell, W.L. Cardiovascular disease prevention and lifestyle interventions: Effectiveness and efficacy. J. Cardiovasc. Nurs. 2003, 18, 245–255.
- 56.Reddy, K.S.; Katan, M.B. Diet, nutrition and the prevention of hypertension and cardiovascular diseases. Public Health Nutr. 2004, 7, 167–186.
- 57.Keys, A. Coronary heart disease in seven countries. Circulation 1970, 41, 186–195.
- 58.Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): A prospective cohort study. Lancet 2017, 390, 2050–2062.
- 59.Perna, M.; Hewlings, S. Saturated fatty acid chain length and risk of cardiovascular disease: A systematic review. Nutrients 2022, 15, 30.
- 60.Lecerf, J.-M. Fatty acids and cardiovascular disease. Nutr. Rev. 2009, 67, 273–283.
- 61.Hu, T.; Wu, Q.; Yao, Q.; Jiang, K.; Yu, J.; Tang, Q. Short-chain fatty acid metabolism and multiple effects on cardiovascular diseases. Ageing Res. Rev. 2022, 81, 101706.
- 62.Lee, Y.-S.; Park, E.-J.; Park, G.-S.; Ko, S.-H.; Park, J.; Lee, Y.-K.; Kim, J.-Y.; Lee, D.; Kang, J.; Lee, H.-J. Lactiplantibacillus plantarum ATG-K2 exerts an anti-obesity effect in high-fat diet-induced obese mice by modulating the gut microbiome. Int. J. Mol. Sci. 2021, 22, 12665.
- 63.Yoo, J.Y.; Sniffen, S.; McGill Percy, K.C.; Pallaval, V.B.; Chidipi, B. Gut dysbiosis and immune system in atherosclerotic cardiovascular disease (ACVD). Microorganisms 2022, 10, 108.
- 64.Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; Van Der Veeken, J.; Deroos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455.
- 65.Nastasi, C.; Candela, M.; Bonefeld, C.M.; Geisler, C.; Hansen, M.; Krejsgaard, T.; Biagi, E.; Andersen, M.H.; Brigidi, P.; Ødum, N. The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci. Rep. 2015, 5, 16148.
- 66.Tian, Q.; Leung, F.P.; Chen, F.M.; Tian, X.Y.; Chen, Z.; Tse, G.; Ma, S.; Wong, W.T. Butyrate protects endothelial function through PPARδ/miR-181b signaling. Pharmacol. Res. 2021, 169, 105681.
- 67.Li, M.; van Esch, B.C.; Henricks, P.A.; Garssen, J.; Folkerts, G. Time and concentration dependent effects of short chain fatty acids on lipopolysaccharide-or tumor necrosis factor α-induced endothelial activation. Front. Pharmacol. 2018, 9, 233.
- 68.Lewis, C.V.; Taylor, W.R. Intestinal barrier dysfunction as a therapeutic target for cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H1227–H1233.
- 69.Zaki, M.H.; Boyd, K.L.; Vogel, P.; Kastan, M.B.; Lamkanfi, M.; Kanneganti, T.-D. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 2010, 32, 379–391.
- 70.Van Hung, T.; Suzuki, T. Dietary fermentable fibers attenuate chronic kidney disease in mice by protecting the intestinal barrier. J. Nutr. 2018, 148, 552–561.
- 71.Keir, M.E.; Yi, T.; Lu, T.T.; Ghilardi, N. The role of IL-22 in intestinal health and disease. J. Exp. Med. 2020, 217, e20192195.
- 72.Yang, W.; Yu, T.; Huang, X.; Bilotta, A.J.; Xu, L.; Lu, Y.; Sun, J.; Pan, F.; Zhou, J.; Zhang, W. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 2020, 11, 4457.
- 73.Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 2015, 17, 662–671.
- 74.Zhao, Y.; Chen, F.; Wu, W.; Sun, M.; Bilotta, A.J.; Yao, S.; Xiao, Y.; Huang, X.; Eaves-Pyles, T.D.; Golovko, G. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. 2018, 11, 752–762.
- 75.Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.; Brummer, R.J. The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119.
- 76.Ziętek, M.; Celewicz, Z.; Szczuko, M. Short-chain fatty acids, maternal microbiota and metabolism in pregnancy. Nutrients 2021, 13, 1244.
- 77.He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int. J. Mol. Sci. 2020, 21, 6356.
- 78.Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472.
- 79.Lu, Y.; Fan, C.; Li, P.; Lu, Y.; Chang, X.; Qi, K. Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating G protein-coupled receptors and gut microbiota. Sci. Rep. 2016, 6, 37589.
- 80.Zhou, H.; Yu, B.; Sun, J.; Liu, Z.; Chen, H.; Ge, L.; Chen, D. Short-chain fatty acids can improve lipid and glucose metabolism independently of the pig gut microbiota. J. Anim. Sci. Biotechnol. 2021, 12, 1–14.
- 81.Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517.
- 82.Yu, Z.; Han, J.; Chen, H.; Wang, Y.; Zhou, L.; Wang, M.; Zhang, R.; Jin, X.; Zhang, G.; Wang, C. Oral supplementation with butyrate improves myocardial ischemia/reperfusion injury via a gut-brain neural circuit. Front. Cardiovasc. Med. 2021, 8, 718674.
- 83.Keshaviah, P.R. The role of acetate in the etiology of symptomatic hypotension. Artif. Organs 1982, 6, 378–384.
- 84.Pagel, M.D.; Ahmad, S.; Vizzo, J.E.; Scribner, B.H. Acetate and bicarbonate fluctuations and acetate intolerance during dialysis. Kidney Int. 1982, 21, 513–518.
- 85.Natarajan, N.; Hori, D.; Flavahan, S.; Steppan, J.; Flavahan, N.A.; Berkowitz, D.E.; Pluznick, J.L. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol. Genom. 2016, 48, 826–834.
- 86.Felizardo, R.J.F.; Watanabe, I.M.; Dardi, P.; Rossoni, L.V.; Câmara, N.O.S. The interplay among gut microbiota, hypertension and kidney diseases: The role of short-chain fatty acids. Pharmacol. Res. 2019, 141, 366–377.
- 87.Hsu, C.-N.; Hou, C.-Y.; Chan, J.Y.; Lee, C.-T.; Tain, Y.-L. Hypertension programmed by perinatal high-fat diet: Effect of maternal gut microbiota-targeted therapy. Nutrients 2019, 11, 2908.
- 88.Greenberger, N.J.; Rodgers, J.; Isselbacher, K. Absorption of medium and long chain triglycerides: Factors influencing their hydrolysis and transport. J. Clin. Investig. 1966, 45, 217–227.
- 89.Acquistapace, S.; Patel, L.; Patin, A.; Forbes-Blom, E.; Cuenoud, B.; Wooster, T.J. Effects of interesterified lipid design on the short/medium chain fatty acid hydrolysis rate and extent (In Vitro). Food Funct. 2019, 10, 4166–4176.
- 90.Shaheen, S.; Kamal, M.; Zhao, C.; Farag, M.A. Fat substitutes and low-calorie fats: A compile of their chemical, nutritional, metabolic and functional properties. Food Rev. Int. 2023, 39, 5501–5527.
- 91.Baral, P.K.; Amin, M.T.; Rashid, M.M.O.; Hossain, M.S. Assessment of polyunsaturated fatty acids on COVID-19-associated risk reduction. Rev. Bras. De Farmacogn. 2022, 32, 50–64.
- 92.Matualatupauw, J.; Bohl, M.; Gregersen, S.; Hermansen, K.; Afman, L. Dietary medium-chain saturated fatty acids induce gene expression of energy metabolism-related pathways in adipose tissue of abdominally obese subjects. Int. J. Obes. 2017, 41, 1348–1354.
- 93.Yue, C.; Li, M.; Li, J.; Han, X.; Zhu, H.; Yu, G.; Cheng, J. Medium-, long-and medium-chain-type structured lipids ameliorate high-fat diet-induced atherosclerosis by regulating inflammation, adipogenesis, and gut microbiota in ApoE−/− mice. Food Funct. 2020, 11, 5142–5155.
- 94.Hecker, M.; Sommer, N.; Voigtmann, H.; Pak, O.; Mohr, A.; Wolf, M.; Vadász, I.; Herold, S.; Weissmann, N.; Morty, R.E. Impact of short‐and medium‐chain fatty acids on mitochondrial function in severe inflammation. Journal of Parenteral and Enteral Nutrition 2014, 38, 587-594.
- 95.Wang, D.; Chen, J.; Sun, H.; Chen, W.; Yang, X. MCFA alleviate H2O2-induced oxidative stress in AML12 cells via the ERK1/2/Nrf2 pathway. Lipids 2022, 57, 153–162.
- 96.Bach, A.C.; Ingenbleek, Y.; Frey, A. The usefulness of dietary medium-chain triglycerides in body weight control: Fact or fancy? J. Lipid Res. 1996, 37, 708–726.
- 97.Miyagawa, Y.; Mori, T.; Goto, K.; Kawahara, I.; Fujiwara-Tani, R.; Kishi, S.; Sasaki, T.; Fujii, K.; Ohmori, H.; Kuniyasu, H. Intake of medium-chain fatty acids induces myocardial oxidative stress and atrophy. Lipids Health Dis. 2018, 17, 1–7.
- 98.Papamandjaris, A.A.; MacDougall, D.E.; Jones, P.J. Medium chain fatty acid metabolism and energy expenditure: Obesity treatment implications. Life Sci. 1998, 62, 1203–1215.
- 99.Ooyama, K.; Wu, J.; Nosaka, N.; Aoyama, T.; Kasai, M. Combined intervention of medium-chain triacylglycerol diet and exercise reduces body fat mass and enhances energy expenditure in rats. J. Nutr. Sci. Vitaminol. 2008, 54, 136–141.
- 100.St-Onge, M.-P.; Jones, P.J. Physiological effects of medium-chain triglycerides: Potential agents in the prevention of obesity. J. Nutr. 2002, 132, 329–332.
- 101.Maher, T.; Deleuse, M.; Thondre, S.; Shafat, A.; Clegg, M.E. A comparison of the satiating properties of medium-chain triglycerides and conjugated linoleic acid in participants with healthy weight and overweight or obesity. Eur. J. Nutr. 2021, 60, 203–215.
- 102.Airhart, S.; Cade, W.T.; Jiang, H.; Coggan, A.R.; Racette, S.B.; Korenblat, K.; Spearie, C.A.; Waller, S.; O’Connor, R.; Bashir, A. A diet rich in medium-chain fatty acids improves systolic function and alters the lipidomic profile in patients with type 2 diabetes: A pilot study. J. Clin. Endocrinol. Metab. 2016, 101, 504–512.
- 103.Yu, J.; Liu, S.; Chen, L.; Wu, B. Combined effects of arsenic and palmitic acid on oxidative stress and lipid metabolism disorder in human hepatoma HepG2 cells. Sci. Total Environ. 2021, 769, 144849.
- 104.Caviglia, J.M.; Gayet, C.; Ota, T.; Hernandez-Ono, A.; Conlon, D.M.; Jiang, H.; Fisher, E.A.; Ginsberg, H.N. Different fatty acids inhibit apoB100 secretion by different pathways: Unique roles for ER stress, ceramide, and autophagy. J. Lipid Res. 2011, 52, 1636–1651.
- 105.Turner, J.D.; Le, N.; Brown, W.V. Effect of changing dietary fat saturation on low-density lipoprotein metabolism in man. Am. J. Physiol. Endocrinol. Metab. 1981, 241, E57–E63.
- 106.Zhang, Y.; Lei, T.; Huang, J.; Wang, S.; Zhou, L.; Yang, Z.; Chen, X. The link between fibroblast growth factor 21 and sterol regulatory element binding protein 1c during lipogenesis in hepatocytes. Mol. Cell. Endocrinol. 2011, 342, 41–47.
- 107.Chu, X.; Liu, L.; Na, L.; Lu, H.; Li, S.; Li, Y.; Sun, C. Sterol Regulatory Element–Binding Protein-1c Mediates Increase of Postprandial Stearic Acid, a Potential Target for Improving Insulin Resistance, in Hyperlipidemia. Diabetes 2013, 62, 561–571.
- 108.Qiu, T.; Yang, X.; Wang, J.; Pan, C.; Chu, X.; Xiong, J.; Xie, J.; Chang, Y.; Wang, C.; Zhang, J. Obesity-induced elevated palmitic acid promotes inflammation and glucose metabolism disorders through GPRs/NF-κB/KLF7 pathway. Nutr. Diabetes 2022, 12, 23.
- 109.Mensink, R.P. Effects of stearic acid on plasma lipid and lipoproteins in humans. Lipids 2005, 40, 1201–1205.
- 110.Gouaref, I.; Bouazza, A.; Abderrhmane, S.A.; Koceir, E.-A. Lipid profile modulates cardiometabolic risk biomarkers including hypertension in people with type-2 diabetes: A focus on unbalanced ratio of plasma polyunsaturated/saturated fatty acids. Molecules 2020, 25, 4315.
- 111.van Rooijen, M.A.; Plat, J.; Blom, W.A.; Zock, P.L.; Mensink, R.P. Dietary stearic acid and palmitic acid do not differently affect ABCA1-mediated cholesterol efflux capacity in healthy men and postmenopausal women: A randomized controlled trial. Clin. Nutr. 2021, 40, 804–811.
- 112.Quan, J.; Liu, J.; Gao, X.; Liu, J.; Yang, H.; Chen, W.; Li, W.; Li, Y.; Yang, W.; Wang, B. Palmitate induces interleukin-8 expression in human aortic vascular smooth muscle cells via T oll-like receptor 4/nuclear factor-κB pathway. J. Diabetes 2014, 6, 33–41.
- 113.Snodgrass, R.G.; Huang, S.; Choi, I.-W.; Rutledge, J.C.; Hwang, D.H. Inflammasome-mediated secretion of IL-1β in human monocytes through TLR2 activation; modulation by dietary fatty acids. J. Immunol. 2013, 191, 4337–4347.
- 114.Cremonini, E.; Oteiza, P.I. (-)-Epicatechin and its metabolites prevent palmitate-induced NADPH oxidase upregulation, oxidative stress and insulin resistance in HepG2 cells. Arch. Biochem. Biophys. 2018, 646, 55–63.
- 115.Roy, A.; Davis, M.; Weinberg, S.; Mallisetty, A.; Hulbert, A.; Weinberg, F.D. Stearic acid induces pro-inflammatory macrophage response important for lung cancer development. Cancer Res. 2024, 84, 176.
- 116.Park, E.-J.; Lee, A.Y.; Park, S.; Kim, J.-H.; Cho, M.-H. Multiple pathways are involved in palmitic acid-induced toxicity. Food Chem. Toxicol. 2014, 67, 26–34.
- 117.Yang, L.; Guan, G.; Lei, L.; Liu, J.; Cao, L.; Wang, X. Oxidative and endoplasmic reticulum stresses are involved in palmitic acid-induced H9c2 cell apoptosis. Biosci. Rep. 2019, 39, BSR20190225.
- 118.Anderson, E.K.; Hill, A.A.; Hasty, A.H. Stearic acid accumulation in macrophages induces TLR4/2-independent inflammation leading to ER stress-mediated apoptosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1687.
- 119.Artwohl, M.; Roden, M.; Waldhäusl, W.; Freudenthaler, A.; Baumgartner-Parzer, S.M. Free fatty acids trigger apoptosis and inhibit cell cycle progression in human vascular endothelial cells. FASEB J. 2004, 18, 146–148.
- 120.Rho, M.-C.; Lee, K.A.; Kim, S.M.; Lee, C.S.; Jang, M.J.; Kim, Y.K.; Lee, H.S.; Choi, Y.H.; Rhim, B.Y.; Kim, K. Sensitization of vascular smooth muscle cell to TNF-α-mediated death in the presence of palmitate. Toxicol. Appl. Pharmacol. 2007, 220, 311–319.
- 121.Shimokawa, H. Primary endothelial dysfunction: Atherosclerosis. J. Mol. Cell. Cardiol. 1999, 31, 23–37.
- 122.Xu, F.; Sun, Y.; Chen, Y.; Sun, Y.; Li, R.; Liu, C.; Zhang, C.; Wang, R.; Zhang, Y. Endothelial cell apoptosis is responsible for the formation of coronary thrombotic atherosclerotic plaques. Tohoku J. Exp. Med. 2009, 218, 25–33.
- 123.Imamura, F.; Sharp, S.J.; Koulman, A.; Schulze, M.B.; Kröger, J.; Griffin, J.L.; Huerta, J.M.; Guevara, M.; Sluijs, I.; Agudo, A. A combination of plasma phospholipid fatty acids and its association with incidence of type 2 diabetes: The EPIC-InterAct case-cohort study. PLoS Med. 2017, 14, e1002409.
- 124.Papandreou, C.; Sala-Vila, A.; Galié, S.; Muralidharan, J.; Estruch, R.; Fitó, M.; Razquin, C.; Corella, D.; Ros, E.; Timiraos, J. Association between fatty acids of blood cell membranes and incidence of coronary heart disease: A case-control study nested in the PREDIMED trial. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 819–825.
- 125.Tao, X.; Liu, L.; Ma, P.; Hu, J.; Ming, Z.; Dang, K.; Zhang, Y.; Li, Y. Association of circulating very long-chain saturated fatty acids with cardiovascular mortality in NHANES 2003-2004, 2011-2012. J. Clin. Endocrinol. Metab. 2024, 109, e633–e645.
- 126.Lemaitre, R.N.; King, I.B. Very long-chain saturated fatty acids and diabetes and cardiovascular disease. Curr. Opin. Lipidol. 2022, 33, 76–82.
- 127.Fretts, A.M.; Imamura, F.; Marklund, M.; Micha, R.; Wu, J.H.; Murphy, R.A.; Chien, K.-L.; McKnight, B.; Tintle, N.; Forouhi, N.G. Associations of circulating very-long-chain saturated fatty acids and incident type 2 diabetes: A pooled analysis of prospective cohort studies. Am. J. Clin. Nutr. 2019, 109, 1216–1223.
- 128.Liu, M.; Zuo, L.; Sun, T.; Wu, Y.; Liu, Y.; Zeng, F.; Chen, Y. Circulating very-long-chain saturated fatty acids were inversely associated with cardiovascular health: A prospective cohort study and meta-analysis. Nutrients 2020, 12, 2709.
- 129.Fretts, A.M.; Mozaffarian, D.; Siscovick, D.S.; King, I.B.; McKnight, B.; Psaty, B.M.; Rimm, E.B.; Sitlani, C.; Sacks, F.M.; Song, X. Associations of plasma phospholipid SFAs with total and cause-specific mortality in older adults differ according to SFA chain length. J. Nutr. 2015, 146, 298.
- 130.Lai, K.Z.H.; Yehia, N.A.; Semnani-Azad, Z.; Mejia, S.B.; Boucher, B.A.; Malik, V.; Bazinet, R.P.; Hanley, A.J. Lifestyle factors associated with circulating very long-chain saturated fatty acids in humans: A systematic review of observational studies. Adv. Nutr. 2023, 14, 99–114.
- 131.Riccardi, G.; Vitale, M.; Vaccaro, O. Are Europeans moving towards dietary habits more suitable for reducing cardiovascular disease risk? Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1857–1860.
- 132.Eilander, A.; Harika, R.K.; Zock, P.L. Intake and sources of dietary fatty acids in Europe: Are current population intakes of fats aligned with dietary recommendations? Eur J Lipid Sci Technol 2015, 117, 1370–1377.
- 133.Giosuè, A.; Calabrese, I.; Vitale, M.; Riccardi, G.; Vaccaro, O. Consumption of Dairy Foods and Cardiovascular Disease: A Systematic Review. Nutrients 2022, 14, 831.
- 134.Alexander, D.D.; Bylsma, L.C.; Vargas, A.J.; Cohen, S.S.; Doucette, A.; Mohamed, M.; Irvin, S.R.; Miller, P.E.; Watson, H.; Fryzek, J.P. Dairy consumption and CVD: A systematic review and meta-analysis. Br. J. Nutr. 2016, 115, 737–750.
- 135.Jakobsen, M.U.; Trolle, E.; Outzen, M.; Mejborn, H.; Grønberg, M.G.; Lyndgaard, C.B.; Stockmarr, A.; Venø, S.K.; Bysted, A. Intake of dairy products and associations with major atherosclerotic cardiovascular diseases: A systematic review and meta-analysis of cohort studies. Sci. Rep. 2021, 11, 1303.
- 136.Gao, X.; Jia, H.Y.; Chen, G.C.; Li, C.Y.; Hao, M. Yogurt Intake Reduces All-Cause and Cardiovascular Disease Mortality: A Meta-Analysis of Eight Prospective Cohort Studies. Chin. J. Integr. Med. 2020, 26, 462–468.
- 137.Wu, L.; Sun, D. Consumption of Yogurt and the Incident Risk of Cardiovascular Disease: A Meta-Analysis of Nine Cohort Studies. Nutrients 2017, 9, 315.
- 138.Al-Shaar, L.; Satija, A.; Wang, D.D.; Rimm, E.B.; Smith-Warner, S.A.; Stampfer, M.J.; Hu, F.B.; Willett, W.C. Red meat intake and risk of coronary heart disease among US men: Prospective cohort study. BMJ 2020, 371, m4141.
- 139.Zheng, Y.; Li, Y.; Satija, A.; Pan, A.; Sotos-Prieto, M.; Rimm, E.; Willett, W.C.; Hu, F.B. Association of changes in red meat consumption with total and cause specific mortality among US women and men: Two prospective cohort studies. BMJ 2019, 365, 12110.
- 140.Abete, I.; Romaguera, D.; Vieira, A.R.; Lopez de Munain, A.; Norat, T. Association between total, processed, red and white meat consumption and all-cause, CVD and IHD mortality: A meta-analysis of cohort studies. Br. J. Nutr. 2014, 112, 762–775.
- 141.Varbo, A.; Nordestgaard, B.G. Remnant cholesterol and ischemic heart disease. Curr. Opin. Lipidol. 2014, 25, 266–273.
- 142.Arora, P.; Kalra, R.; Callas, P.W.; Alexander, K.S.; Zakai, N.A.; Wadley, V.; Arora, G.; Kissela, B.M.; Judd, S.E.; Cushman, M. Lipoprotein(a) and Risk of Ischemic Stroke in the REGARDS Study. Arter. Thromb. Vasc. Biol. 2019, 39, 810–818.
- 143.Ramos Meyers, G.; Samouda, H.; Bohn, T. Short chain fatty acid metabolism in relation to gut microbiota and genetic variability. Nutrients 2022, 14, 5361.
- 144.Huang, L.; Gao, L.; Chen, C. Role of medium-chain fatty acids in healthy metabolism: A clinical perspective. Trends Endocrinol. Metab. 2021, 32, 351–366.
- 145.Mickiewicz, A.; Marlęga-Linert, J.; Czapiewska, M.; Marcinkowska, M.; Krzesińska, A.; Kuchta, A.; Fijałkowski, M.; Gruchała, M.; Mika, A. Fatty acid analysis in serum of patients with elevated lipoprotein (a) and cardiovascular disease undergoing lipoprotein apheresis. J. Clin. Lipidol. 2024, 18, e197–e206.
- 146.Dougkas, A.; Hobbs, D. The role of milk and dairy products in the development of obesity and cardiometabolic disease. In Handbook of Eating and Drinking; Springer: Cham, Switzerland, 2020.
- 147.Mozaffarian, D.; Clarke, R. Quantitative effects on cardiovascular risk factors and coronary heart disease risk of replacing partially hydrogenated vegetable oils with other fats and oils. Eur. J. Clin. Nutr. 2009, 63, S22–S33.
- 148.Lenighan, Y.M.; McNulty, B.A.; Roche, H.M. Dietary fat composition: Replacement of saturated fatty acids with PUFA as a public health strategy, with an emphasis on α-linolenic acid. Proc. Nutr. Soc. 2019, 78, 234–245.
- 149.Livingstone, K.M.; Lovegrove, J.A.; Givens, D.I. The impact of substituting SFA in dairy products with MUFA or PUFA on CVD risk: Evidence from human intervention studies. Nutr. Res. Rev. 2012, 25, 193–206.
- 150.Pan, A.; Sun, Q.; Bernstein, A.M.; Schulze, M.B.; Manson, J.E.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Red meat consumption and mortality: Results from 2 prospective cohort studies. Arch. Intern. Med. 2012, 172, 555–563.
- 151.Yannakoulia, M.; Yiannakouris, N.; Melistas, L.; Kontogianni, M.D.; Malagaris, I.; Mantzoros, C.S. A dietary pattern characterized by high consumption of whole-grain cereals and low-fat dairy products and low consumption of refined cereals is positively associated with plasma adiponectin levels in healthy women. Metabolism 2008, 57, 824–830.
How to Cite
Huang, X.; Tadese, D. A.; Lu, Q.; Lai, R. Diverse Mechanisms of Saturated Fatty Acids in Cardiovascular Disease. Health and Metabolism 2025, 2 (4), 9. https://doi.org/10.53941/hm.2025.100032.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


