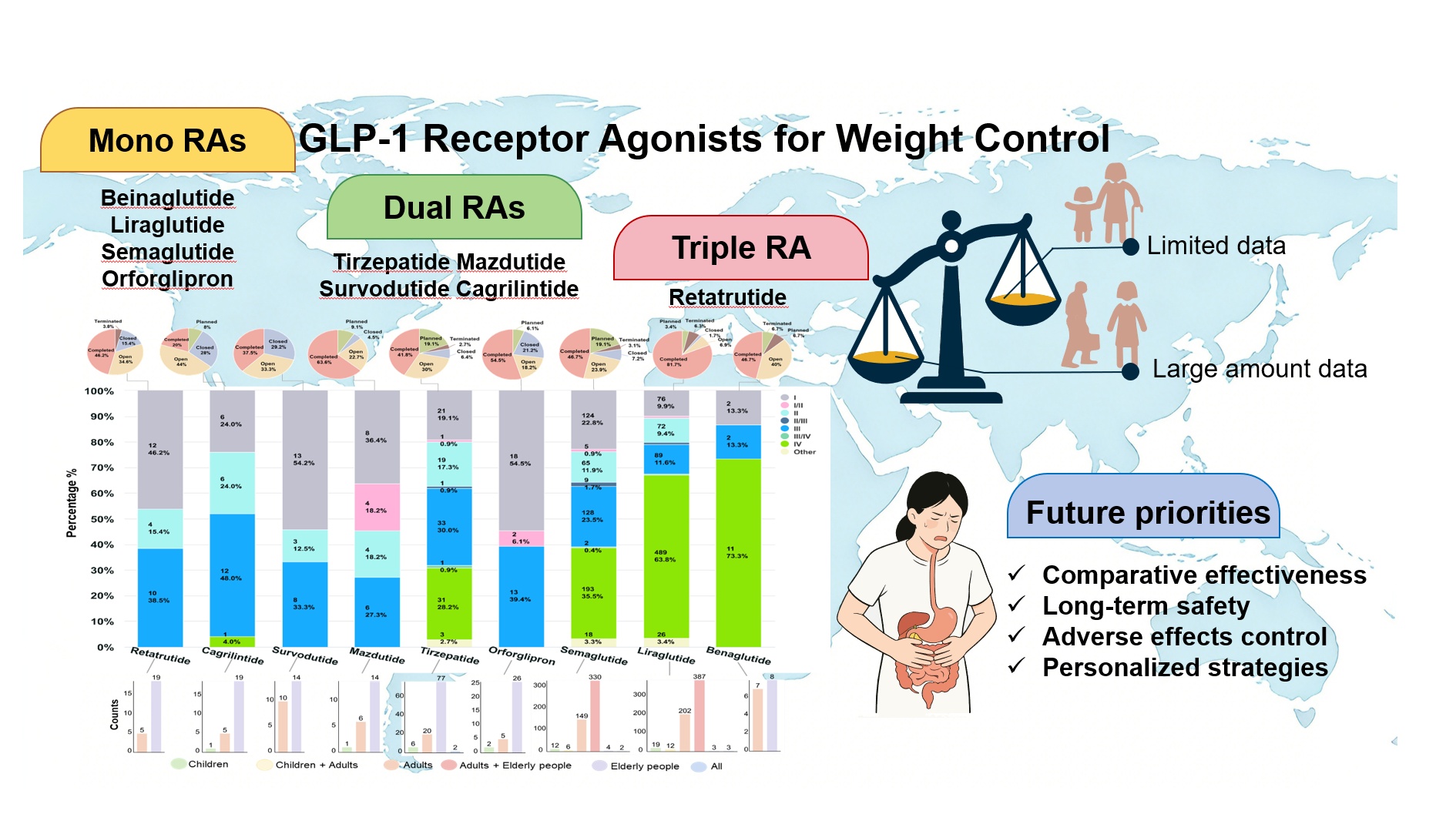
Background: The rising global prevalence of obesity presents a major public health concern. Glucagon-like peptide-1 receptor agonists (GLP-1RAs) emerge as promising therapeutic agents, showing substantial efficacy in weight reduction in clinical settings. Methods: This review synthesizes data from 1565 clinical trials (Phases I–IV) sourced from the Trialtrove database (November 2024), covering nine GLP-1RAs: mono-agonists (beinaglutide, liraglutide, semaglutide, orforglipron), dual agonists (tirzepatide, mazdutide, survodutide, cagrilintide), and the triple agonist retatrutide. We analyzed trial phase distributions and updated current evidence on safety and efficacy across populations, emphasizing both therapeutic promise and current limitations, particularly the scarcity of long-term and comparative effectiveness data as well as associated adverse effects. Future research must prioritize comparative effectiveness, long-term safety, and personalized strategies for GLP-1RAs, addressing evidence gaps in special populations and treatment individualization. Conclusion: This review synthesizes current evidence on the safety and effectiveness of GLP-1RAs in weight management and potential cardiovascular protection. Further trials are imperative to clarify long-term outcomes, individual variability, and specific adverse effects across diverse populations.
- Open Access
- Review
GLP-1 Receptor Agonists for Weight Control: Emerging Insights from Clinical Trials and Future Perspectives
- Jiayao Lv 1,†,
- Shiyi Zhang 1,†,
- Yizhe Qu 2,
- Yousen Cao 3,
- Jiayi Zhang 1,
- Xiaoshuang Dai 4,
- Lin Shi 1,*
Author Information
Received: 12 Jun 2025 | Revised: 07 Oct 2025 | Accepted: 22 Oct 2025 | Published: 16 Jan 2026
Abstract
Graphical Abstract
Keywords
GLP-1 receptor agonists | clinical trials | weight loss | adverse events | personalized medicine
References
- 1.
Lingvay, I.; Cohen, R.V.; Roux, C.W.; Sumithran, P. Obesity in adults. Lancet 2024, 404, 972–987. https://doi.org/10.1016/S0140-6736(24)01210-8.
- 2.
Yanovski, S.Z.; Yanovski, J.A. Approach to Obesity Treatment in Primary Care: A Review. JAMA Intern. Med. 2024, 184, 818–829. https://doi.org/10.1001/jamainternmed.2023.8526.
- 3.
Ussher, J.R.; Drucker, D.J. Glucagon-like peptide 1 receptor agonists: Cardiovascular benefits and mechanisms of action. Nat. Rev. Cardiol. 2023, 20, 463–474. https://doi.org/10.1038/s41569-023-00849-3.
- 4.
Nogueiras, R.; Nauck, M.A.; Tschöp, M.H. Gut hormone co-agonists for the treatment of obesity: From bench to bedside. Nat. Metab. 2023, 5, 933–944. https://doi.org/10.1038/s42255-023-00812-z.
- 5.
Kokkorakis, M.; Chakhtoura, M.; Rhayem, C.; Al Rifai, J.; Ghezzawi, M.; Valenzuela-Vallejo, L.; Mantzoros, C.S. Emerging pharmacotherapies for obesity: A systematic review. Pharmacol. Rev. 2025, 77, 100002. https://doi.org/10.1124/pharmrev.123.001045.
- 6.
Ansari, H.U.H.; Qazi, S.U.; Sajid, F.; Altaf, Z.; Ghazanfar, S.; Naveed, N.; Ashfaq, A.S.; Siddiqui, A.H.; Iqbal, H.; Qazi, S. Efficacy and Safety of Glucagon-Like Peptide-1 Receptor Agonists on Body Weight and Cardiometabolic Parameters in Individuals with Obesity and without Diabetes: A Systematic Review and Meta-Analysis. Endocr. Pract. 2024, 30, 160–171. https://doi.org/10.1016/j.eprac.2023.11.007.
- 7.
Nicze, M.; Dec, A.; Borówka, M.; Krzyżak, D.; Bołdys, A.; Bułdak, Ł.; Okopień, B. Molecular Mechanisms behind Obesity and Their Potential Exploitation in Current and Future Therapy. Int. J. Mol. Sci. 2024, 25, 8202. https://doi.org/10.3390/ijms25158202.
- 8.
Gudzune, K.A.; Kushner, R.F. Medications for Obesity: A Review. JAMA 2024, 332, 571–584. https://doi.org/10.1001/jama.2024.10816.
- 9.
Weihrauch-Blüher, S.; Schwarz, P.; Klusmann, J.-H. Childhood obesity: Increased risk for cardiometabolic disease and cancer in adulthood. Metab. Clin. Exp. 2019, 92, 147–152. https://doi.org/10.1016/j.metabol.2018.12.001.
- 10.
Shi, Q.; Wang, Y.; Hao, Q.; Vandvik, P.O.; Guyatt, G.; Li, J.; Chen, Z.; Xu, S.; Shen, Y.; Ge, L.; et al. Pharmacotherapy for adults with overweight and obesity: A systematic review and network meta-analysis of randomised controlled trials. Lancet 2024, 403, e21–e31. https://doi.org/10.1016/S0140-6736(24)00351-9.
- 11.
Rubino, D.M.; Greenway, F.L.; Khalid, U.; O’Neil, P.M.; Rosenstock, J.; Sørrig, R.; Wadden, T.A.; Wizert, A.; Garvey, W.T.; Investigators, S. Effect of Weekly Subcutaneous Semaglutide vs Daily Liraglutide on Body Weight in Adults With Overweight or Obesity Without Diabetes: The STEP 8 Randomized Clinical Trial. JAMA 2022, 327, 138–150. https://doi.org/10.1001/jama.2021.23619.
- 12.
Mok, J.; Adeleke, M.O.; Brown, A.; Magee, C.G.; Firman, C.; Makahamadze, C.; Jassil, F.C.; Marvasti, P.; Carnemolla, A.; Devalia, K.; et al. Safety and Efficacy of Liraglutide, 3.0 mg, Once Daily vs Placebo in Patients With Poor Weight Loss Following Metabolic Surgery: The BARI-OPTIMISE Randomized Clinical Trial. JAMA Surg. 2023, 158, 1003–1011. https://doi.org/10.1001/jamasurg.2023.2930.
- 13.
Mashayekhi, M.; Nian, H.; Mayfield, D.; Devin, J.K.; Gamboa, J.L.; Yu, C.; Silver, H.J.; Niswender, K.; Luther, J.M.; Brown, N.J. Weight Loss–Independent Effect of Liraglutide on Insulin Sensitivity in Individuals with Obesity and Prediabetes. Diabetes 2023, 73, 38–50. https://doi.org/10.2337/db23-0356.
- 14.
Foghsgaard, S.; Vedtofte, L.; Andersen, E.S.; Bahne, E.; Andreasen, C.; Sørensen, A.L.; Forman, J.L.; Mathiesen, E.R.; Svare, J.A.; Clausen, T.D.; et al. Liraglutide treatment for the prevention of glucose tolerance deterioration in women with prior gestational diabetes mellitus: A 52-week randomized controlled clinical trial. Diabetes Obes. Metab. 2024, 26, 201–214. https://doi.org/10.1111/dom.15306.
- 15.
Kelly Aaron, S.; Auerbach, P.; Barrientos-Perez, M.; Gies, I.; Hale Paula, M.; Marcus, C.; Mastrandrea Lucy, D.; Prabhu, N.; Arslanian, S. A Randomized, Controlled Trial of Liraglutide for Adolescents with Obesity. N. Engl. J. Med. 2020, 382, 2117–2128. https://doi.org/10.1056/NEJMoa1916038.
- 16.
Fox Claudia, K.; Barrientos-Pérez, M.; Bomberg Eric, M.; Dcruz, J.; Gies, I.; Harder-Lauridsen Nina, M.; Jalaludin Muhammad, Y.; Sahu, K.; Weimers, P.; Zueger, T.; et al. Liraglutide for Children 6 to <12 Years of Age with Obesity—A Randomized Trial. N. Engl. J. Med. 2025, 392, 555–565. https://doi.org/10.1056/NEJMoa2407379.
- 17.
Ryan, D.H.; Lingvay, I.; Deanfield, J.; Kahn, S.E.; Barros, E.; Burguera, B.; Colhoun, H.M.; Cercato, C.; Dicker, D.; Horn, D.B.; et al. Long-term weight loss effects of semaglutide in obesity without diabetes in the SELECT trial. Nat. Med. 2024, 30, 2049–2057. https://doi.org/10.1038/s41591-024-02996-7.
- 18.
Kelsey, M.; Newby, L.K. In higher-risk, statin-intolerant adults with diabetes, bempedoic acid reduced MACE at a median 3 y. Ann. Intern. Med. 2024, 177, JC39. https://doi.org/10.7326/J24-0016.
- 19.
Kadowaki, T.; Isendahl, J.; Khalid, U.; Lee, S.Y.; Nishida, T.; Ogawa, W.; Tobe, K.; Yamauchi, T.; Lim, S. Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): A randomised, double-blind, double-dummy, placebo-controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2022, 10, 193–206. https://doi.org/10.1016/S2213-8587(22)00008-0.
- 20.
Weghuber, D.; Barrett, T.; Barrientos-Pérez, M.; Gies, I.; Hesse, D.; Jeppesen Ole, K.; Kelly Aaron, S.; Mastrandrea Lucy, D.; Sørrig, R.; Arslanian, S. Once-Weekly Semaglutide in Adolescents with Obesity. N. Engl. J. Med. 2022, 387, 2245–2257. https://doi.org/10.1056/NEJMoa2208601.
- 21.
Knop, F.K.; Aroda, V.R.; do Vale, R.D.; Holst-Hansen, T.; Laursen, P.N.; Rosenstock, J.; Rubino, D.M.; Garvey, W.T. Oral semaglutide 50 mg taken once per day in adults with overweight or obesity (OASIS 1): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 402, 705–719. https://doi.org/10.1016/S0140-6736(23)01185-6.
- 22.
Wilding John, P.H.; Batterham Rachel, L.; Calanna, S.; Davies, M.; Van Gaal Luc, F.; Lingvay, I.; McGowan Barbara, M.; Rosenstock, J.; Tran Marie, T.D.; Wadden Thomas, A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. https://doi.org/10.1056/NEJMoa2032183.
- 23.
Zhang, F.; Chen, Z.; Wu, D.; Tian, L.; Chen, Q.; Ye, Y.; Chen, W.; Wu, X.; Wu, P.; Yuan, W.; et al. Recombinant human GLP-1 beinaglutide regulates lipid metabolism of adipose tissues in diet-induced obese mice. iScience 2021, 24. https://doi.org/10.1016/j.isci.2021.103382.
- 24.
Chen, K.; Chen, L.; Shan, Z.; Wang, G.; Qu, S.; Qin, G.; Yu, X.; Xin, W.; Hsieh, T.-h.; Mu, Y. Beinaglutide for weight management in Chinese individuals with overweight or obesity: A phase 3 randomized controlled clinical study. Diabetes Obes. Metab. 2024, 26, 690–698. https://doi.org/10.1111/dom.15360.
- 25.
Gao, L.; Huang, H.; Zhang, L.; Zhang, N.; Fu, Y.; Zhu, D.; Bi, Y.; Feng, W. Comparison of Beinaglutide Versus Metformin for Weight Loss in Overweight and Obese Non-diabetic Patients. Exp Clin Endocrinol Diabetes 2022, 130, 358–367. https://doi.org/10.1055/a-1608-0345.
- 26.
Pratt, E.; Ma, X.; Liu, R.; Robins, D.; Coskun, T.; Sloop, K.W.; Haupt, A.; Benson, C. Orforglipron (LY3502970), a novel, oral non-peptide glucagon-like peptide-1 receptor agonist: A Phase 1b, multicentre, blinded, placebo-controlled, randomized, multiple-ascending-dose study in people with type 2 diabetes. Diabetes Obes. Metab. 2023, 25, 2642–2649. https://doi.org/10.1111/dom.15150.
- 27.
Wharton, S.; Blevins, T.; Connery, L.; Rosenstock, J.; Raha, S.; Liu, R.; Ma, X.; Mather Kieren, J.; Haupt, A.; Robins, D.; et al. Daily Oral GLP-1 Receptor Agonist Orforglipron for Adults with Obesity. N. Engl. J. Med. 2023, 389, 877–888. https://doi.org/10.1056/NEJMoa2302392.
- 28.
Frias, J.P.; Hsia, S.; Eyde, S.; Liu, R.; Ma, X.; Konig, M.; Kazda, C.; Mather, K.J.; Haupt, A.; Pratt, E.; et al. Efficacy and safety of oral orforglipron in patients with type 2 diabetes: A multicentre, randomised, dose-response, phase 2 study. Lancet 2023, 402, 472–483. https://doi.org/10.1016/S0140-6736(23)01302-8.
- 29.
Dahl, D.; Onishi, Y.; Norwood, P.; Huh, R.; Bray, R.; Patel, H.; Rodríguez, Á. Effect of Subcutaneous Tirzepatide vs Placebo Added to Titrated Insulin Glargine on Glycemic Control in Patients with Type 2 Diabetes: The SURPASS-5 Randomized Clinical Trial. JAMA 2022, 327, 534–545. https://doi.org/10.1001/jama.2022.0078.
- 30.
Rosenstock, J.; Frías, J.P.; Rodbard, H.W.; Tofé, S.; Sears, E.; Huh, R.; Fernández Landó, L.; Patel, H. Tirzepatide vs Insulin Lispro Added to Basal Insulin in Type 2 Diabetes: The SURPASS-6 Randomized Clinical Trial. JAMA 2023, 330, 1631–1640. https://doi.org/10.1001/jama.2023.20294.
- 31.
Del Prato, S.; Kahn, S.E.; Pavo, I.; Weerakkody, G.J.; Yang, Z.; Doupis, J.; Aizenberg, D.; Wynne, A.G.; Riesmeyer, J.S.; Heine, R.J.; et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): A randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet 2021, 398, 1811–1824. https://doi.org/10.1016/S0140-6736(21)02188-7.
- 32.
Jastreboff Ania, M.; Aronne Louis, J.; Ahmad Nadia, N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck Mathijs, C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. https://doi.org/10.1056/NEJMoa2206038.
- 33.
Garvey, W.T.; Frias, J.P.; Jastreboff, A.M.; le Roux, C.W.; Sattar, N.; Aizenberg, D.; Mao, H.; Zhang, S.; Ahmad, N.N.; Bunck, M.C.; et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): A double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2023, 402, 613–626. https://doi.org/10.1016/S0140-6736(23)01200-X.
- 34.
Wadden, T.A.; Chao, A.M.; Machineni, S.; Kushner, R.; Ard, J.; Srivastava, G.; Halpern, B.; Zhang, S.; Chen, J.; Bunck, M.C.; et al. Tirzepatide after intensive lifestyle intervention in adults with overweight or obesity: The SURMOUNT-3 phase 3 trial. Nat. Med. 2023, 29, 2909–2918. https://doi.org/10.1038/s41591-023-02597-w.
- 35.
Aronne, L.J.; Sattar, N.; Horn, D.B.; Bays, H.E.; Wharton, S.; Lin, W.-Y.; Ahmad, N.N.; Zhang, S.; Liao, R.; Bunck, M.C.; et al. Continued Treatment With Tirzepatide for Maintenance of Weight Reduction in Adults With Obesity: The SURMOUNT-4 Randomized Clinical Trial. JAMA 2024, 331, 38–48. https://doi.org/10.1001/jama.2023.24945.
- 36.
Kadowaki, T.; Kiyosue, A.; Shingaki, T.; Oura, T.; Yokote, K. Efficacy and safety of once-weekly tirzepatide in Japanese patients with obesity disease (SURMOUNT-J): A multicentre, randomised, double-blind, placebo-controlled phase 3 trial. Lancet Diabetes Endocrinol. 2025, 13, 384–396. https://doi.org/10.1016/S2213-8587(24)00377-2.
- 37.
Zhao, L.; Cheng, Z.; Lu, Y.; Liu, M.; Chen, H.; Zhang, M.; Wang, R.; Yuan, Y.; Li, X. Tirzepatide for Weight Reduction in Chinese Adults With Obesity: The SURMOUNT-CN Randomized Clinical Trial. JAMA 2024, 332, 551–560. https://doi.org/10.1001/jama.2024.9217.
- 38.
Nalisa, D.L.; Cuboia, N.; Dyab, E.; Jackson, I.L.; Felix, H.J.; Shoki, P.; Mubiana, M.; Oyedeji-Amusa, M.; Azevedo, L.; Jiang, H. Efficacy and safety of Mazdutide on weight loss among diabetic and non-diabetic patients: A systematic review and meta-analysis of randomized controlled trials. Front. Endocrinol. 2024, 15, 1309118.
- 39.
le Roux, C.W.; Steen, O.; Lucas, K.J.; Startseva, E.; Unseld, A.; Hennige, A.M. Glucagon and GLP-1 receptor dual agonist survodutide for obesity: A randomised, double-blind, placebo-controlled, dose-finding phase 2 trial. Lancet Diabetes Endocrinol. 2024, 12, 162–173. https://doi.org/10.1016/S2213-8587(23)00356-X.
- 40.
Blüher, M.; Rosenstock, J.; Hoefler, J.; Manuel, R.; Hennige, A.M. Dose–response effects on HbA1c and bodyweight reduction of survodutide, a dual glucagon/GLP-1 receptor agonist, compared with placebo and open-label semaglutide in people with type 2 diabetes: A randomised clinical trial. Diabetologia 2024, 67, 470–482. https://doi.org/10.1007/s00125-023-06053-9.
- 41.
Enebo, L.B.; Berthelsen, K.K.; Kankam, M.; Lund, M.T.; Rubino, D.M.; Satylganova, A.; Lau, D.C.W. Safety, tolerability, pharmacokinetics, and pharmacodynamics of concomitant administration of multiple doses of cagrilintide with semaglutide 2·4 mg for weight management: A randomised, controlled, phase 1b trial. Lancet 2021, 397, 1736–1748. https://doi.org/10.1016/S0140-6736(21)00845-X.
- 42.
Lau, D.C.W.; Erichsen, L.; Francisco, A.M.; Satylganova, A.; le Roux, C.W.; McGowan, B.; Pedersen, S.D.; Pietiläinen, K.H.; Rubino, D.; Batterham, R.L. Once-weekly cagrilintide for weight management in people with overweight and obesity: A multicentre, randomised, double-blind, placebo-controlled and active-controlled, dose-finding phase 2 trial. Lancet 2021, 398, 2160–2172. https://doi.org/10.1016/S0140-6736(21)01751-7.
- 43.
Frias, J.P.; Deenadayalan, S.; Erichsen, L.; Knop, F.K.; Lingvay, I.; Macura, S.; Mathieu, C.; Pedersen, S.D.; Davies, M. Efficacy and safety of co-administered once-weekly cagrilintide 2·4 mg with once-weekly semaglutide 2·4 mg in type 2 diabetes: A multicentre, randomised, double-blind, active-controlled, phase 2 trial. Lancet 2023, 402, 720–730. https://doi.org/10.1016/S0140-6736(23)01163-7.
- 44.
Dutta, D.; Nagendra, L.; Harish, B.G.; Sharma, M.; Joshi, A.; Hathur, B.; Kamrul-Hasan, A.B.M. Efficacy and Safety of Cagrilintide Alone and in Combination with Semaglutide (Cagrisema) as Anti-Obesity Medications: A Systematic Review and Meta-Analysis. Indian J. Endocrinol. Metab. 2024, 28, 436–444.
- 45.
Kaur, M.; Misra, S. A review of an investigational drug retatrutide, a novel triple agonist agent for the treatment of obesity. Eur. J. Clin. Pharmacol. 2024, 80, 669–676. https://doi.org/10.1007/s00228-024-03646-0.
- 46.
Abdul-Rahman, T.; Roy, P.; Ahmed, F.K.; Mueller-Gomez, J.L.; Sarkar, S.; Garg, N.; Femi-Lawal, V.O.; Wireko, A.A.; Thaalibi, H.I.; Hashmi, M.U.; et al. The power of three: Retatrutide's role in modern obesity and diabetes therapy. Eur. J. Pharmacol. 2024, 985, 177095. https://doi.org/10.1016/j.ejphar.2024.177095.
- 47.
Doggrell, S.A. Is retatrutide (LY3437943), a GLP-1, GIP, and glucagon receptor agonist a step forward in the treatment of diabetes and obesity? Expert Opin. Investig. Drugs 2023, 32, 355–359. https://doi.org/10.1080/13543784.2023.2206560.
- 48.
Ray, A. Retatrutide: A triple incretin receptor agonist for obesity management. Expert Opin. Investig. Drugs 2023, 32, 1003–1008. https://doi.org/10.1080/13543784.2023.2276754.
- 49.
Coskun, T.; Urva, S.; Roell, W.C.; Qu, H.; Loghin, C.; Moyers, J.S.; O’Farrell, L.S.; Briere, D.A.; Sloop, K.W.; Thomas, M.K.; et al. LY3437943, a novel triple glucagon, GIP, and GLP-1 receptor agonist for glycemic control and weight loss: From discovery to clinical proof of concept. Cell Metab. 2022, 34, 1234–1247. https://doi.org/10.1016/j.cmet.2022.07.013.
- 50.
Jastreboff Ania, M.; Kaplan Lee, M.; Frías Juan, P.; Wu, Q.; Du, Y.; Gurbuz, S.; Coskun, T.; Haupt, A.; Milicevic, Z.; Hartman Mark, L. Triple–Hormone-Receptor Agonist Retatrutide for Obesity—A Phase 2 Trial. N. Engl. J. Med. 2023, 389, 514–526. https://doi.org/10.1056/NEJMoa2301972.
- 51.
Pan, X.-H.; Tan, B.; Chin, Y.H.; Lee, E.C.Z.; Kong, G.; Chong, B.; Kueh, M.; Khoo, C.M.; Mehta, A.; Majety, P.; et al. Efficacy and safety of tirzepatide, GLP-1 receptor agonists, and other weight loss drugs in overweight and obesity: A network meta-analysis. Obesity 2024, 32, 840–856. https://doi.org/10.1002/oby.24002.
- 52.
le Roux, C.W.; Hankosky, E.R.; Wang, D.; Malik, R.; Yu, M.; Hickey, A.; Kan, H.; Bunck, M.C.; Stefanski, A.; Garcia-Perez, L.-E.; et al. Tirzepatide 10 and 15 mg compared with semaglutide 2.4 mg for the treatment of obesity: An indirect treatment comparison. Diabetes Obes. Metab. 2023, 25, 2626–2633. https://doi.org/10.1111/dom.15148.
- 53.
Wu, T.; Zhang, Y.; Shi, Y.; Yu, K.; Zhao, M.; Liu, S.; Zhao, Z. Safety of Glucagon-Like Peptide-1 Receptor Agonists: A Real-World Study Based on the US FDA Adverse Event Reporting System Database. Clin. Drug Investig. 2022, 42, 965–975. https://doi.org/10.1007/s40261-022-01202-1.
- 54.
Winkler, G.; Hajós, P.; Kiss, J.T. A glükagonszerű peptid-1 (GLP1) és a gyomor-bél rendszer. GLP1-receptor-agonisták–túlértékelt gyomor-, elfelejtődött bél- („ileal brake”) hatás? Orvosi Hetil. OH 2019, 160, 1927–1934. https://doi.org/10.1556/650.2019.31615.
- 55.
Huang, X.; Wu, M.; Huang, B.; Zhang, Y.; Yusta, B.; Baggio, L.L.; Koehler, J.; Holland, D.; Cao, X.; Pinnell, L.J.; et al. Gastrointestinal adverse events associated with GLP-1 receptor agonists in metabolic dysfunction-associated steatotic liver disease (MASLD): A systematic review and meta-analysis. Front. Med. 2025, 12, 1509947. https://doi.org/10.3389/fmed.2025.1509947.
- 56.
Khan, S.S.; Ndumele, C.E.; Kazi, D.S. Discontinuation of Glucagon-Like Peptide-1 Receptor Agonists. JAMA 2025, 333, 113–114. https://doi.org/10.1001/jama.2024.22284.
- 57.
Horowitz, M.; Aroda, V.R.; Han, J.; Hardy, E.; Rayner, C.K. Upper and/or lower gastrointestinal adverse events with glucagon-like peptide-1 receptor agonists: Incidence and consequences. Diabetes Obes. Metab. 2017, 19, 672–681. https://doi.org/10.1111/dom.12872.
- 58.
Do, D.; Lee, T.; Peasah, S.K.; Good, C.B.; Inneh, A.; Patel, U. GLP-1 Receptor Agonist Discontinuation Among Patients With Obesity and/or Type 2 Diabetes. JAMA Netw. Open 2024, 7, e2413172. https://doi.org/10.1001/jamanetworkopen.2024.13172.
- 59.
Bettge, K.; Kahle, M.; Abd El Aziz, M.S.; Meier, J.J.; Nauck, M.A. Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon-like peptide-1 receptor agonists: A systematic analysis of published clinical trials. Diabetes Obes. Metab. 2017, 19, 336–347. https://doi.org/10.1111/dom.12824.
- 60.
Osei, S.P.; Akomaning, E.; Florut, T.F.; Sodhi, M.; Lacy, B.E.; Aldhaleei, W.A.; Bhagavathula, A.S. Gastrointestinal Safety Assessment of GLP-1 Receptor Agonists in the US: A Real-World Adverse Events Analysis from the FAERS Database. Diagnostics 2024, 14, 2829. https://doi.org/10.3390/diagnostics14242829.
- 61.
Xie, Y.; Choi, T.; Al-Aly, Z. Mapping the effectiveness and risks of GLP-1 receptor agonists. Nat. Med. 2025, 31, 951–962. https://doi.org/10.1038/s41591-024-03412-w.
- 62.
Holst, J.J. GLP-1 physiology in obesity and development of incretin-based drugs for chronic weight management. Nat. Metab. 2024, 6, 1866–1885. https://doi.org/10.1038/s42255-024-01113-9.
- 63.
Freeman, J.S. Optimizing outcomes for GLP-1 agonists. J. Osteopath. Med. 2011, 111, eS15–eS20.
- 64.
Wilding, J.P.H.; Batterham, R.L.; Davies, M.; Van Gaal, L.F.; Kandler, K.; Konakli, K.; Lingvay, I.; McGowan, B.M.; Oral, T.K.; Rosenstock, J.; et al. Weight regain and cardiometabolic effects after withdrawal of semaglutide: The STEP 1 trial extension. Diabetes Obes. Metab. 2022, 24, 1553–1564. https://doi.org/10.1111/dom.14725.
- 65.
Lundgren Julie, R.; Janus, C.; Jensen Simon, B.K.; Juhl Christian, R.; Olsen Lisa, M.; Christensen Rasmus, M.; Svane Maria, S.; Bandholm, T.; Bojsen-Møller Kirstine, N.; Blond Martin, B.; et al. Healthy Weight Loss Maintenance with Exercise, Liraglutide, or Both Combined. N. Engl. J. Med. 2021, 384, 1719–1730. https://doi.org/10.1056/NEJMoa2028198.

This work is licensed under a Creative Commons Attribution 4.0 International License.


