Henipaviruses are lethal zoonotic paramyxoviruses with high mortality, increasing geographic reach, and a growing number of identified species and variants. No vaccines or therapeutics are licensed for human use, creating an urgent need for effective interventions. This review highlights recent advances in neutralizing antibodies against the viral attachment (G) and fusion (F) glycoproteins, focusing on structural and mechanistic insights. Lead monoclonal antibodies target conserved epitopes and block viral entry through receptor interference or fusion inhibition. Key challenges, including narrow treatment windows and viral escape, underscore the need for rationally designed combinations and broad-spectrum, escape-resistant candidates. By consolidating current progress, we outline strategies for developing antibody therapeutics with high translational impact, serving as a first line of defense in outbreak settings and as a complementary intervention to vaccines for comprehensive henipavirus management.
- Open Access
- Review
Progress in Henipavirus Neutralizing Antibody for Therapeutic Development
- Junping Hong 1,2,
- Yuanteng Zhao 1,2,
- Ethan H. Zhou 3,
- Eleni Kourlas 1,2,
- Zhangfei Shen 1,2,
- Kai Xu 1,2,4,*
Author Information
Received: 08 Aug 2025 | Revised: 15 Oct 2025 | Accepted: 28 Oct 2025 | Published: 22 Jan 2026
Abstract
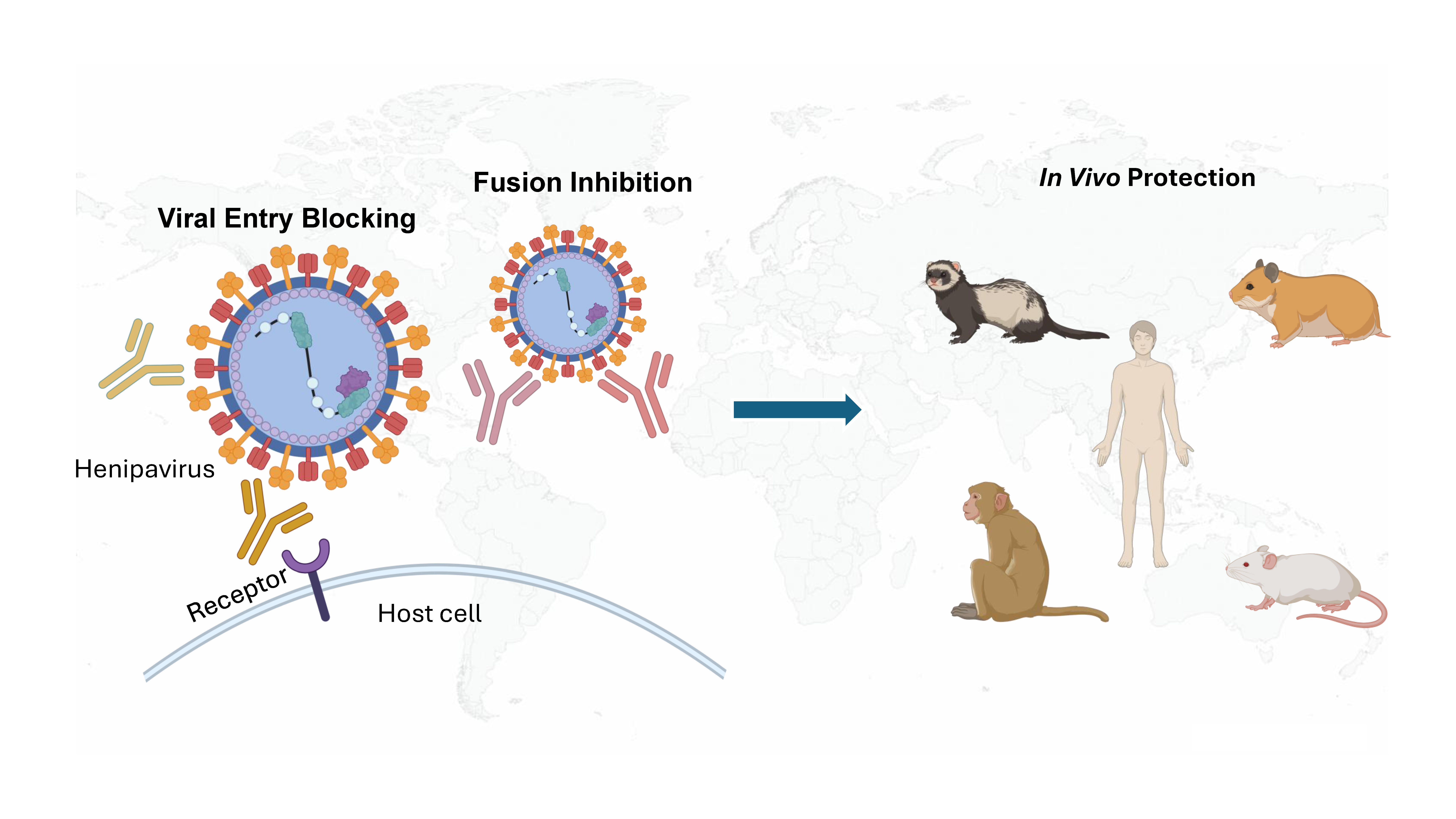
Graphical Abstract
Keywords
References
- 1.
Eaton, B.T.; Broder, C.C.; Middleton, D.; Wang, L.-F. Hendra and Nipah viruses: Different and dangerous. Nat. Rev. Microbiol. 2006, 4, 23–35. https://doi.org/10.1038/nrmicro1323.
- 2.
Quarleri, J.; Galvan, V.; Delpino, M.V. Henipaviruses: An expanding global public health concern? GeroScience 2022, 44, 2447–2459. https://doi.org/10.1007/s11357-022-00670-9.
- 3.
Wang, L.F.; Yu, M.; Hansson, E.; Pritchard, L.I.; Shiell, B.; Michalski, W.P.; Eaton, B.T. The exceptionally large genome of Hendra virus: Support for creation of a new genus within the family Paramyxoviridae. J. Virol. 2000, 74, 9972–9979. https://doi.org/10.1128/jvi.74.21.9972-9979.2000.
- 4.
Harcourt, B.H.; Tamin, A.; Halpin, K.; Ksiazek, T.G.; Rollin, P.E.; Bellini, W.J.; Rota, P.A. Molecular characterization of the polymerase gene and genomic termini of Nipah virus. Virology 2001, 287, 192–201. https://doi.org/10.1006/viro.2001.1026.
- 5.
Ker, D.-S.; Jenkins, H.T.; Greive, S.J.; Antson, A.A. CryoEM structure of the Nipah virus nucleocapsid assembly. PLoS Pathog. 2021, 17, e1009740. https://doi.org/10.1371/journal.ppat.1009740.
- 6.
Species List: Paramyxoviridae. Available online: https://ictv.global/report/chapter/paramyxoviridae/taxonomy/paramyxoviridae (accessed on 1 December 2026)
- 7.
Murray, K.; Rogers, R.; Selvey, L.; Selleck, P.; Hyatt, A.; Gould, A.; Gleeson, L.; Hooper, P.; Westbury, H. A novel morbillivirus pneumonia of horses and its transmission to humans. Emerg. Infect. Dis. 1995, 1, 31–33. https://doi.org/10.3201/eid0101.950107.
- 8.
Murray, K.; Selleck, P.; Hooper, P.; Hyatt, A.; Gould, A.; Gleeson, L.; Westbury, H.; Hiley, L.; Selvey, L.; Rodwell, B. A morbillivirus that caused fatal disease in horses and humans. Science 1995, 268, 94–97. https://doi.org/10.1126/science.7701348.
- 9.
Paton, N.I.; Leo, Y.S.; Zaki, S.R.; Auchus, A.P.; Lee, K.E.; Ling, A.E.; Chew, S.K.; Ang, B.; Rollin, P.E.; Umapathi, T.; et al. Outbreak of Nipah-virus infection among abattoir workers in Singapore. Lancet 1999, 354, 1253–1256. https://doi.org/10.1016/S0140-6736(99)04379-2.
- 10.
Chua, K.B.; Bellini, W.J.; Rota, P.A.; Harcourt, B.H.; Tamin, A.; Lam, S.K.; Ksiazek, T.G.; Rollin, P.E.; Zaki, S.R.; Shieh, W.; et al. Nipah virus: A recently emergent deadly paramyxovirus. Science 2000, 288, 1432–1435. https://doi.org/10.1126/science.288.5470.1432.
- 11.
Chua, K.B. Nipah virus outbreak in Malaysia. J. Clin. Virol. 2003, 26, 265–275. https://doi.org/10.1016/s1386-6532(02)00268-8.
- 12.
Khan, S.; Akbar, S.M.F.; Mahtab, M.A.; Uddin, M.N.; Rashid, M.M.; Yahiro, T.; Hashimoto, T.; Kimitsuki, K.; Nishizono, A. Twenty-five years of Nipah outbreaks in Southeast Asia: A persistent threat to global health. IJID Reg. 2024, 13, 100434. https://doi.org/10.1016/j.ijregi.2024.100434.
- 13.
Drexler, J.F.; Corman, V.M.; Müller, M.A.; Maganga, G.D.; Vallo, P.; Binger, T.; Gloza-Rausch, F.; Cottontail, V.M.; Rasche, A.; Yordanov, S.; et al. Bats host major mammalian paramyxoviruses. Nat. Commun. 2012, 3, 796. https://doi.org/10.1038/ncomms1796.
- 14.
Marsh, G.A.; de Jong, C.; Barr, J.A.; Tachedjian, M.; Smith, C.; Middleton, D.; Yu, M.; Todd, S.; Foord, A.J.; Haring, V.; et al. Cedar virus: A novel Henipavirus isolated from Australian bats. PLoS Pathog. 2012, 8, e1002836. https://doi.org/10.1371/journal.ppat.1002836.
- 15.
Wu, Z.; Yang, L.; Yang, F.; Ren, X.; Jiang, J.; Dong, J.; Sun, L.; Zhu, Y.; Zhou, H.; Jin, Q. Novel Henipa-like virus, Mojiang Paramyxovirus, in rats, China, 2012. Emerg. Infect. Dis. 2014, 20, 1064–1066. https://doi.org/10.3201/eid2006.131022.
- 16.
Kuang, G.; Yang, T.; Yang, W.; Wang, J.; Pan, H.; Pan, Y.; Gou, Q.Y.; Wu, W.C.; Wang, J.; Yang, L.; et al. Infectome analysis of bat kidneys from Yunnan province, China, reveals novel henipaviruses related to Hendra and Nipah viruses and prevalent bacterial and eukaryotic microbes. PLoS Pathog. 2025, 21, e1013235. https://doi.org/10.1371/journal.ppat.1013235.
- 17.
Lee, S.H.; Kim, K.; Kim, J.; No, J.S.; Park, K.; Budhathoki, S.; Lee, S.H.; Lee, J.; Cho, S.H.; Cho, S.; et al. Discovery and Genetic Characterization of Novel Paramyxoviruses Related to the Genus Henipavirus in Crocidura Species in the Republic of Korea. Viruses 2021, 13, 2020. https://doi.org/10.3390/v13102020.
- 18.
Kaza, B.; Aguilar, H.C. Pathogenicity and virulence of henipaviruses. Virulence 2023, 14, 2273684. https://doi.org/10.1080/21505594.2023.2273684.
- 19.
Taylor, J.; Thompson, K.; Annand, E.J.; Massey, P.D.; Bennett, J.; Eden, J.-S.; Horsburgh, B.A.; Hodgson, E.; Wood, K.; Kerr, J.; et al. Novel variant Hendra virus genotype 2 infection in a horse in the greater Newcastle region, New South Wales, Australia. One Health 2022, 15, 100423. https://doi.org/10.1016/j.onehlt.2022.100423.
- 20.
Parry, R.H.; Yamada, K.Y.H.; Hood, W.R.; Zhao, Y.; Lu, J.Y.; Seluanov, A.; Gorbunova, V.; Modhiran, N.; Watterson, D.; Isaacs, A. Henipavirus in northern short-tailed shrew, Alabama, USA. Emerg. Infect. Dis. 2025, 31, 392–394. https://doi.org/10.3201/eid3102.241155.
- 21.
Zhang, X.A.; Li, H.; Jiang, F.C.; Zhu, F.; Zhang, Y.F.; Chen, J.J.; Tan, C.W.; Anderson, D.E.; Fan, H.; Dong, L.Y.; et al. A Zoonotic Henipavirus in Febrile Patients in China. N. Engl. J. Med. 2022, 387, 470–472. https://doi.org/10.1056/NEJMc2202705.
- 22.
Hernández, L.H.A.; da Paz, T.Y.B.; Silva, S.P.D.; Silva, F.S.D.; Barros, B.C.V.; Nunes, B.T.D.; Casseb, L.M.N.; Medeiros, D.B.A.; Vasconcelos, P.; Cruz, A.C.R. First Genomic Evidence of a Henipa-like Virus in Brazil. Viruses 2022, 14, 2167. https://doi.org/10.3390/v14102167.
- 23.
Vanmechelen, B.; Meurs, S.; Horemans, M.; Loosen, A.; Joly Maes, T.; Laenen, L.; Vergote, V.; Koundouno, F.R.; Magassouba, N.; Konde, M.K.; et al. The characterization of multiple novel paramyxoviruses highlights the diverse nature of the subfamily Orthoparamyxovirinae. Virus Evol 2022, 8, veac061. https://doi.org/10.1093/ve/veac061.
- 24.
Madera, S.; Kistler, A.; Ranaivoson, H.C.; Ahyong, V.; Andrianiaina, A.; Andry, S.; Raharinosy, V.; Randriambolamanantsoa, T.H.; Ravelomanantsoa, N.A.F.; Tato, C.M.; et al. Discovery and Genomic Characterization of a Novel Henipavirus, Angavokely Virus, from Fruit Bats in Madagascar. J. Virol. 2022, 96, e0092122. https://doi.org/10.1128/jvi.00921-22.
- 25.
Clayton, B.A.; Wang, L.F.; Marsh, G.A. Henipaviruses: An updated review focusing on the pteropid reservoir and features of transmission: Henipaviruses: The pteropid reservoir and features of transmission. Zoonoses Public Health 2013, 60, 69–83. https://doi.org/10.1111/j.1863-2378.2012.01501.x.
- 26.
Epstein, J.H.; Anthony, S.J.; Islam, A.; Kilpatrick, A.M.; Ali Khan, S.; Balkey, M.D.; Ross, N.; Smith, I.; Zambrana-Torrelio, C.; Tao, Y.; et al. Nipah virus dynamics in bats and implications for spillover to humans. Proc. Natl. Acad. Sci. USA 2020, 117, 29190–29201. https://doi.org/10.1073/pnas.2000429117.
- 27.
Garbuglia, A.R.; Lapa, D.; Pauciullo, S.; Raoul, H.; Pannetier, D. Nipah virus: An overview of the current status of diagnostics and their role in preparedness in endemic countries. Viruses 2023, 15, 2062. https://doi.org/10.3390/v15102062.
- 28.
Rahman, M.Z.; Islam, M.M.; Hossain, M.E.; Rahman, M.M.; Islam, A.; Siddika, A.; Hossain, M.S.S.; Sultana, S.; Islam, A.; Rahman, M.; et al. Genetic diversity of Nipah virus in Bangladesh. Int. J. Infect. Dis. 2021, 102, 144–151. https://doi.org/10.1016/j.ijid.2020.10.041.
- 29.
Halpin, K.; Rota, P.A. A Review of Hendra Virus and Nipah Virus Infections in Man and Other Animals. In Zoonoses: Infections Affecting Humans and Animals, Sing, A., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1493–1508.
- 30.
Singh, R.K.; Dhama, K.; Chakraborty, S.; Tiwari, R.; Natesan, S.; Khandia, R.; Munjal, A.; Vora, K.S.; Latheef, S.K.; Karthik, K.; et al. Nipah virus: Epidemiology, pathology, immunobiology and advances in diagnosis, vaccine designing and control strategies—A comprehensive review. Vet. Q. 2019, 39, 26–55. https://doi.org/10.1080/01652176.2019.1580827.
- 31.
O’Sullivan, J.D.; Allworth, A.M.; Paterson, D.L.; Snow, T.M.; Boots, R.; Gleeson, L.J.; Gould, A.R.; Hyatt, A.D.; Bradfield, J. Fatal encephalitis due to novel paramyxovirus transmitted from horses. Lancet 1997, 349, 93–95. https://doi.org/10.1016/s0140-6736(96)06162-4.
- 32.
Wong, K.T.; Shieh, W.-J.; Kumar, S.; Norain, K.; Abdullah, W.; Guarner, J.; Goldsmith, C.S.; Chua, K.B.; Lam, S.K.; Tan, C.T.; et al. Nipah virus infection: Pathology and pathogenesis of an emerging paramyxoviral zoonosis. Am. J. Pathol. 2002, 161, 2153–2167. https://doi.org/10.1016/S0002-9440(10)64493-8.
- 33.
Enchéry, F.; Horvat, B. Understanding the interaction between henipaviruses and their natural host, fruit bats: Paving the way toward control of highly lethal infection in humans. Int. Rev. Immunol. 2017, 36, 108–121. https://doi.org/10.1080/08830185.2016.1255883.
- 34.
Wang, Z.; Amaya, M.; Addetia, A.; Dang, H.V.; Reggiano, G.; Yan, L.; Hickey, A.C.; DiMaio, F.; Broder, C.C.; Veesler, D. Architecture and antigenicity of the Nipah virus attachment glycoprotein. Science 2022, 375, 1373–1378. https://doi.org/10.1126/science.abm5561.
- 35.
Guo, Y.; Wu, S.; Li, W.; Yang, H.; Shi, T.; Ju, B.; Zhang, Z.; Yan, R. The cryo-EM structure of homotetrameric attachment glycoprotein from langya henipavirus. Nat. Commun. 2024, 15, 812. https://doi.org/10.1038/s41467-024-45202-5.
- 36.
Laing, E.D.; Navaratnarajah, C.K.; Cheliout Da Silva, S.; Petzing, S.R.; Xu, Y.; Sterling, S.L.; Marsh, G.A.; Wang, L.F.; Amaya, M.; Nikolov, D.B.; et al. Structural and functional analyses reveal promiscuous and species specific use of ephrin receptors by Cedar virus. Proc. Natl. Acad. Sci. USA 2019, 116, 20707–20715. https://doi.org/10.1073/pnas.1911773116.
- 37.
Pager, C.T.; Dutch, R.E. Cathepsin L is involved in proteolytic processing of the Hendra virus fusion protein. J. Virol. 2005, 79, 12714–12720. https://doi.org/10.1128/JVI.79.20.12714-12720.2005.
- 38.
Diederich, S.; Thiel, L.; Maisner, A. Role of endocytosis and cathepsin-mediated activation in Nipah virus entry. Virology 2008, 375, 391–400. https://doi.org/10.1016/j.virol.2008.02.019.
- 39.
Diederich, S.; Sauerhering, L.; Weis, M.; Altmeppen, H.; Schaschke, N.; Reinheckel, T.; Erbar, S.; Maisner, A. Activation of the Nipah virus fusion protein in MDCK cells is mediated by cathepsin B within the endosome-recycling compartment. J. Virol. 2012, 86, 3736–3745. https://doi.org/10.1128/JVI.06628-11.
- 40.
Xu, K.; Chan, Y.P.; Bradel-Tretheway, B.; Akyol-Ataman, Z.; Zhu, Y.; Dutta, S.; Yan, L.; Feng, Y.; Wang, L.F.; Skiniotis, G.; et al. Crystal Structure of the Pre-fusion Nipah Virus Fusion Glycoprotein Reveals a Novel Hexamer-of-Trimers Assembly. PLoS Pathog. 2015, 11, e1005322. https://doi.org/10.1371/journal.ppat.1005322.
- 41.
Wong, J.J.; Paterson, R.G.; Lamb, R.A.; Jardetzky, T.S. Structure and stabilization of the Hendra virus F glycoprotein in its prefusion form. Proc. Natl. Acad. Sci. USA 2016, 113, 1056–1061. https://doi.org/10.1073/pnas.1523303113.
- 42.
Wong, J.J.W.; Young, T.A.; Zhang, J.; Liu, S.; Leser, G.P.; Komives, E.A.; Lamb, R.A.; Zhou, Z.H.; Salafsky, J.; Jardetzky, T.S. Monomeric ephrinB2 binding induces allosteric changes in Nipah virus G that precede its full activation. Nat. Commun. 2017, 8, 781. https://doi.org/10.1038/s41467-017-00863-3.
- 43.
Liu, Q.; Stone, J.A.; Bradel-Tretheway, B.; Dabundo, J.; Benavides Montano, J.A.; Santos-Montanez, J.; Biering, S.B.; Nicola, A.V.; Iorio, R.M.; Lu, X.; et al. Unraveling a three-step spatiotemporal mechanism of triggering of receptor-induced Nipah virus fusion and cell entry. PLoS Pathog. 2013, 9, e1003770. https://doi.org/10.1371/journal.ppat.1003770.
- 44.
Liu, Q.; Bradel-Tretheway, B.; Monreal, A.I.; Saludes, J.P.; Lu, X.; Nicola, A.V.; Aguilar, H.C. Nipah virus attachment glycoprotein stalk C-terminal region links receptor binding to fusion triggering. J. Virol. 2015, 89, 1838–1850. https://doi.org/10.1128/jvi.02277-14.
- 45.
Lizbeth Reyes Zamora, J.; Ortega, V.; Johnston, G.P.; Li, J.; André, N.M.; Abrrey Monreal, I.; Contreras, E.M.; Whittaker, G.R.; Aguilar, H.C. Third Helical Domain of the Nipah Virus Fusion Glycoprotein Modulates both Early and Late Steps in the Membrane Fusion Cascade. J. Virol. 2020, 94. https://doi.org/10.1128/jvi.00644-20.
- 46.
Negrete, O.A.; Wolf, M.C.; Aguilar, H.C.; Enterlein, S.; Wang, W.; Mühlberger, E.; Su, S.V.; Bertolotti-Ciarlet, A.; Flick, R.; Lee, B. Two key residues in ephrinB3 are critical for its use as an alternative receptor for Nipah virus. PLoS Pathog. 2006, 2, e7. https://doi.org/10.1371/journal.ppat.0020007.
- 47.
Xu, K.; Chan, Y.P.; Rajashankar, K.R.; Khetawat, D.; Yan, L.; Kolev, M.V.; Broder, C.C.; Nikolov, D.B. New insights into the Hendra virus attachment and entry process from structures of the virus G glycoprotein and its complex with Ephrin-B2. PLoS ONE 2012, 7, e48742. https://doi.org/10.1371/journal.pone.0048742.
- 48.
Lee, B.; Pernet, O.; Ahmed, A.A.; Zeltina, A.; Beaty, S.M.; Bowden, T.A. Molecular recognition of human ephrinB2 cell surface receptor by an emergent African henipavirus. Proc. Natl. Acad. Sci. USA 2015, 112, E2156–E2165. https://doi.org/10.1073/pnas.1501690112.
- 49.
Li, Y.; Li, R.; Wang, M.; Liu, Y.; Yin, Y.; Zai, X.; Song, X.; Chen, Y.; Xu, J.; Chen, W. Fc-Based Recombinant Henipavirus Vaccines Elicit Broad Neutralizing Antibody Responses in Mice. Viruses 2020, 12, 480. https://doi.org/10.3390/v12040480.
- 50.
Wang, C.; Li, M.; Wang, Y.; Ding, Q.; Fan, S.; Lan, J. Structural insights into the Langya virus attachment glycoprotein. Structure 2024, 32, 1090–1098.e1093. https://doi.org/10.1016/j.str.2024.05.003.
- 51.
Broder, C.C.; Xu, K.; Nikolov, D.B.; Zhu, Z.; Dimitrov, D.S.; Middleton, D.; Pallister, J.; Geisbert, T.W.; Bossart, K.N.; Wang, L.-F. A treatment for and vaccine against the deadly Hendra and Nipah viruses. Antivir. Res. 2013, 100, 8–13. https://doi.org/10.1016/j.antiviral.2013.06.012.
- 52.
Geisbert, T.W.; Bobb, K.; Borisevich, V.; Geisbert, J.B.; Agans, K.N.; Cross, R.W.; Prasad, A.N.; Fenton, K.A.; Yu, H.; Fouts, T.R.; et al. A single dose investigational subunit vaccine for human use against Nipah virus and Hendra virus. NPJ Vaccines 2021, 6, 23. https://doi.org/10.1038/s41541-021-00284-w.
- 53.
Halpin, K.; Graham, K.; Durr, P.A. Sero-monitoring of horses demonstrates the equivac® HeV Hendra virus vaccine to be highly effective in inducing neutralising antibody titres. Vaccines 2021, 9, 731. https://doi.org/10.3390/vaccines9070731.
- 54.
Playford, E.G.; Munro, T.; Mahler, S.M.; Elliott, S.; Gerometta, M.; Hoger, K.L.; Jones, M.L.; Griffin, P.; Lynch, K.D.; Carroll, H.; et al. Safety, tolerability, pharmacokinetics, and immunogenicity of a human monoclonal antibody targeting the G glycoprotein of henipaviruses in healthy adults: A first-in-human, randomised, controlled, phase 1 study. Lancet Infect. Dis. 2020, 20, 445–454. https://doi.org/10.1016/S1473-3099(19)30634-6.
- 55.
Steffen, D.L.; Xu, K.; Nikolov, D.B.; Broder, C.C. Henipavirus mediated membrane fusion, virus entry and targeted therapeutics. Viruses 2012, 4, 280–308. https://doi.org/10.3390/v4020280.
- 56.
Zhu, Z.; Dimitrov, A.S.; Bossart, K.N.; Crameri, G.; Bishop, K.A.; Choudhry, V.; Mungall, B.A.; Feng, Y.-R.; Choudhary, A.; Zhang, M.-Y.; et al. Potent neutralization of Hendra and Nipah viruses by human monoclonal antibodies. J. Virol. 2006, 80, 891–899. https://doi.org/10.1128/JVI.80.2.891-899.2006.
- 57.
Zhu, Z.; Bossart, K.N.; Bishop, K.A.; Crameri, G.; Dimitrov, A.S.; McEachern, J.A.; Feng, Y.; Middleton, D.; Wang, L.F.; Broder, C.C.; et al. Exceptionally potent cross-reactive neutralization of Nipah and Hendra viruses by a human monoclonal antibody. J. Infect. Dis. 2008, 197, 846–853. https://doi.org/10.1086/528801.
- 58.
Bossart, K.N.; Zhu, Z.; Middleton, D.; Klippel, J.; Crameri, G.; Bingham, J.; McEachern, J.A.; Green, D.; Hancock, T.J.; Chan, Y.-P.; et al. A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute nipah virus infection. PLoS Pathog. 2009, 5, e1000642. https://doi.org/10.1371/journal.ppat.1000642.
- 59.
Geisbert, T.W.; Daddario-DiCaprio, K.M.; Hickey, A.C.; Smith, M.A.; Chan, Y.-P.; Wang, L.-F.; Mattapallil, J.J.; Geisbert, J.B.; Bossart, K.N.; Broder, C.C. Development of an acute and highly pathogenic nonhuman primate model of Nipah virus infection. PLoS ONE 2010, 5, e10690. https://doi.org/10.1371/journal.pone.0010690.
- 60.
Rockx, B.; Bossart, K.N.; Feldmann, F.; Geisbert, J.B.; Hickey, A.C.; Brining, D.; Callison, J.; Safronetz, D.; Marzi, A.; Kercher, L.; et al. A novel model of lethal Hendra virus infection in African green monkeys and the effectiveness of ribavirin treatment. J. Virol. 2010, 84, 9831–9839. https://doi.org/10.1128/JVI.01163-10.
- 61.
Bossart, K.N.; Geisbert, T.W.; Feldmann, H.; Zhu, Z.; Feldmann, F.; Geisbert, J.B.; Yan, L.; Feng, Y.R.; Brining, D.; Scott, D.; et al. A neutralizing human monoclonal antibody protects african green monkeys from hendra virus challenge. Sci. Transl. Med. 2011, 3, 105ra103. https://doi.org/10.1126/scitranslmed.3002901.
- 62.
Geisbert, T.W.; Mire, C.E.; Geisbert, J.B.; Chan, Y.P.; Agans, K.N.; Feldmann, F.; Fenton, K.A.; Zhu, Z.; Dimitrov, D.S.; Scott, D.P.; et al. Therapeutic treatment of Nipah virus infection in nonhuman primates with a neutralizing human monoclonal antibody. Sci. Transl. Med. 2014, 6, 242ra282. https://doi.org/10.1126/scitranslmed.3008929.
- 63.
Mire, C.E.; Satterfield, B.A.; Geisbert, J.B.; Agans, K.N.; Borisevich, V.; Yan, L.; Chan, Y.-P.; Cross, R.W.; Fenton, K.A.; Broder, C.C.; et al. Pathogenic differences between nipah virus Bangladesh and Malaysia strains in primates: Implications for antibody therapy. Sci. Rep. 2016, 6, 30916. https://doi.org/10.1038/srep30916.
- 64.
Xu, K.; Rockx, B.; Xie, Y.; DeBuysscher, B.L.; Fusco, D.L.; Zhu, Z.; Chan, Y.P.; Xu, Y.; Luu, T.; Cer, R.Z.; et al. Crystal structure of the Hendra virus attachment G glycoprotein bound to a potent cross-reactive neutralizing human monoclonal antibody. PLoS Pathog. 2013, 9, e1003684. https://doi.org/10.1371/journal.ppat.1003684.
- 65.
Bowden, T.A.; Aricescu, A.R.; Gilbert, R.J.C.; Grimes, J.M.; Jones, E.Y.; Stuart, D.I. Structural basis of Nipah and Hendra virus attachment to their cell-surface receptor ephrin-B2. Nat. Struct. Mol. Biol. 2008, 15, 567–572. https://doi.org/10.1038/nsmb.1435.
- 66.
Dong, J.; Cross, R.W.; Doyle, M.P.; Kose, N.; Mousa, J.J.; Annand, E.J.; Borisevich, V.; Agans, K.N.; Sutton, R.; Nargi, R.; et al. Potent Henipavirus Neutralization by Antibodies Recognizing Diverse Sites on Hendra and Nipah Virus Receptor Binding Protein. Cell 2020, 183, 1536–1550 e1517. https://doi.org/10.1016/j.cell.2020.11.023.
- 67.
Doyle, M.P.; Kose, N.; Borisevich, V.; Binshtein, E.; Amaya, M.; Nagel, M.; Annand, E.J.; Armstrong, E.; Bombardi, R.; Dong, J.; et al. Cooperativity mediated by rationally selected combinations of human monoclonal antibodies targeting the henipavirus receptor binding protein. Cell Rep. 2021, 36, 109628. https://doi.org/10.1016/j.celrep.2021.109628.
- 68.
Chen, L.; Sun, M.; Zhang, H.; Zhang, X.; Yao, Y.; Li, M.; Li, K.; Fan, P.; Zhang, H.; Qin, Y.; et al. Potent human neutralizing antibodies against Nipah virus derived from two ancestral antibody heavy chains. Nat. Commun. 2024, 15, 2987. https://doi.org/10.1038/s41467-024-47213-8.
- 69.
Fan, P.; Sun, M.; Zhang, X.; Zhang, H.; Liu, Y.; Yao, Y.; Li, M.; Fang, T.; Sun, B.; Chen, Z.; et al. A potent Henipavirus cross-neutralizing antibody reveals a dynamic fusion-triggering pattern of the G-tetramer. Nat. Commun. 2024, 15, 4330. https://doi.org/10.1038/s41467-024-48601-w.
- 70.
Wang, Y.; Sun, Y.; Shen, Z.; Wang, C.; Qian, J.; Mao, Q.; Wang, Y.; Song, W.; Kong, Y.; Zhan, C.; et al. Fully human single-domain antibody targeting a highly conserved cryptic epitope on the Nipah virus G protein. Nat. Commun. 2024, 15, 6892. https://doi.org/10.1038/s41467-024-51066-6.
- 71.
Guillaume, V.; Contamin, H.; Loth, P.; Grosjean, I.; Courbot, M.C.G.; Deubel, V.; Buckland, R.; Wild, T.F. Antibody prophylaxis and therapy against Nipah virus infection in hamsters. J. Virol. 2006, 80, 1972–1978. https://doi.org/10.1128/JVI.80.4.1972-1978.2006.
- 72.
Mire, C.E.; Chan, Y.-P.; Borisevich, V.; Cross, R.W.; Yan, L.; Agans, K.N.; Dang, H.V.; Veesler, D.; Fenton, K.A.; Geisbert, T.W.; et al. A cross-reactive humanized monoclonal antibody targeting fusion glycoprotein function protects ferrets against lethal nipah virus and Hendra virus infection. J. Infect. Dis. 2020, 221, S471–S479. https://doi.org/10.1093/infdis/jiz515.
- 73.
Avanzato, V.A.; Bushmaker, T.; Oguntuyo, K.Y.; Yinda, C.K.; Duyvesteyn, H.M.E.; Stass, R.; Meade-White, K.; Rosenke, R.; Thomas, T.; van Doremalen, N.; et al. A monoclonal antibody targeting the Nipah virus fusion glycoprotein apex imparts protection from disease. J. Virol. 2024, 98, e0063824. https://doi.org/10.1128/jvi.00638-24.
- 74.
Zeitlin, L.; Cross, R.W.; Woolsey, C.; West, B.R.; Borisevich, V.; Agans, K.N.; Prasad, A.N.; Deer, D.J.; Stuart, L.; McCavitt-Malvido, M.; et al. Therapeutic administration of a cross-reactive mAb targeting the fusion glycoprotein of Nipah virus protects nonhuman primates. Sci. Transl. Med. 2024, 16, eadl2055. https://doi.org/10.1126/scitranslmed.adl2055.
- 75.
Ren, Y.; Fan, P.; Zhang, X.; Fang, T.; Chen, Z.; Yao, Y.; Chi, X.; Zhang, G.; Zhao, X.; Sun, B.; et al. Potent Cross-neutralizing Antibodies Reveal Vulnerabilities of Henipavirus Fusion Glycoprotein. Adv. Sci. 2025, 12, e2501996. https://doi.org/10.1002/advs.202501996.
- 76.
Isaacs, A.; Nieto, G.V.; Zhang, X.; Modhiran, N.; Barr, J.; Thakur, N.; Low, Y.S.; Parry, R.H.; Barnes, J.B.; Jara, R.; et al. A protective bispecific antibody targets both Nipah virus surface glycoproteins and limits viral escape. bioRxiv 2025. https://doi.org/10.1101/2025.03.11.642517.
- 77.
Huang, X.; Li, Y.; Li, R.; Wang, S.; Yang, L.; Wang, S.; Yin, Y.; Zai, X.; Zhang, J.; Xu, J. Nipah virus attachment glycoprotein ectodomain delivered by type 5 adenovirus vector elicits broad immune response against NiV and HeV. Front. Cell. Infect. Microbiol. 2023, 13, 1180344. https://doi.org/10.3389/fcimb.2023.1180344.
- 78.
Borisevich, V.; Lee, B.; Hickey, A.; DeBuysscher, B.; Broder, C.C.; Feldmann, H.; Rockx, B. Escape From Monoclonal Antibody Neutralization Affects Henipavirus Fitness In Vitro and In Vivo. J. Infect. Dis. 2016, 213, 448–455. https://doi.org/10.1093/infdis/jiv449.
- 79.
Wang, Z.; Dang, H.V.; Amaya, M.; Xu, Y.; Yin, R.; Yan, L.; Hickey, A.C.; Annand, E.J.; Horsburgh, B.A.; Reid, P.A.; et al. Potent monoclonal antibody-mediated neutralization of a divergent Hendra virus variant. Proc. Natl. Acad. Sci. USA 2022, 119, e2122769119. https://doi.org/10.1073/pnas.2122769119.
- 80.
Hickey, A.C. Defining the Antigenic Structure of the Henipavirus Attachment (G) Glycoprotein: Implications for the Fusion Mechanism. Ph.D. Thesis, Uniformed Services University of the Health Sciences, Bethesda,MD, USA, 2009.
- 81.
Zhou, D.; Cheng, R.; Yao, Y.; Zhang, G.; Li, X.; Wang, B.; Wang, Y.; Yu, F.; Yang, S.; Liu, H.; et al. An attachment glycoprotein nanoparticle elicits broadly neutralizing antibodies and protects against lethal Nipah virus infection. NPJ Vaccines 2024, 9, 158. https://doi.org/10.1038/s41541-024-00954-5.
- 82.
Aguilar, H.C.; Matreyek, K.A.; Choi, D.Y.; Filone, C.M.; Young, S.; Lee, B. Polybasic KKR motif in the cytoplasmic tail of Nipah virus fusion protein modulates membrane fusion by inside-out signaling. J. Virol. 2007, 81, 4520–4532. https://doi.org/10.1128/jvi.02205-06.
- 83.
Avanzato, V.A.; Oguntuyo, K.Y.; Escalera-Zamudio, M.; Gutierrez, B.; Golden, M.; Kosakovsky Pond, S.L.; Pryce, R.; Walter, T.S.; Seow, J.; Doores, K.J.; et al. A structural basis for antibody-mediated neutralization of Nipah virus reveals a site of vulnerability at the fusion glycoprotein apex. Proc. Natl. Acad. Sci. USA 2019, 116, 25057–25067. https://doi.org/10.1073/pnas.1912503116.
- 84.
Dang, H.V.; Cross, R.W.; Borisevich, V.; Bornholdt, Z.A.; West, B.R.; Chan, Y.P.; Mire, C.E.; Da Silva, S.C.; Dimitrov, A.S.; Yan, L.; et al. Broadly neutralizing antibody cocktails targeting Nipah virus and Hendra virus fusion glycoproteins. Nat. Struct. Mol. Biol. 2021, 28, 426–434. https://doi.org/10.1038/s41594-021-00584-8.
- 85.
Byrne, P.O.; Fisher, B.E.; Ambrozak, D.R.; Blade, E.G.; Tsybovsky, Y.; Graham, B.S.; McLellan, J.S.; Loomis, R.J. Structural basis for antibody recognition of vulnerable epitopes on Nipah virus F protein. Nat. Commun. 2023, 14, 1494. https://doi.org/10.1038/s41467-023-36995-y.
- 86.
Chan, Y.P.; Lu, M.; Dutta, S.; Yan, L.; Barr, J.; Flora, M.; Feng, Y.R.; Xu, K.; Nikolov, D.B.; Wang, L.F.; et al. Biochemical, conformational, and immunogenic analysis of soluble trimeric forms of henipavirus fusion glycoproteins. J. Virol. 2012, 86, 11457–11471. https://doi.org/10.1128/jvi.01318-12.
- 87.
Dang, H.V.; Chan, Y.P.; Park, Y.J.; Snijder, J.; Da Silva, S.C.; Vu, B.; Yan, L.; Feng, Y.R.; Rockx, B.; Geisbert, T.W.; et al. An antibody against the F glycoprotein inhibits Nipah and Hendra virus infections. Nat. Struct. Mol. Biol. 2019, 26, 980–987. https://doi.org/10.1038/s41594-019-0308-9.
- 88.
Loomis, R.J.; Stewart-Jones, G.B.E.; Tsybovsky, Y.; Caringal, R.T.; Morabito, K.M.; McLellan, J.S.; Chamberlain, A.L.; Nugent, S.T.; Hutchinson, G.B.; Kueltzo, L.A.; et al. Structure-Based Design of Nipah Virus Vaccines: A Generalizable Approach to Paramyxovirus Immunogen Development. Front. Immunol. 2020, 11, 842. https://doi.org/10.3389/fimmu.2020.00842.
- 89.
Pigeaud, D.D.; Geisbert, T.W.; Woolsey, C. Animal Models for Henipavirus Research. Viruses 2023, 15, 1980. https://doi.org/10.3390/v15101980.
- 90.
Findlay-Wilson, S.; Flett, L.; Salguero, F.J.; Ruedas-Torres, I.; Fotheringham, S.; Easterbrook, L.; Graham, V.; Dowall, S. Establishment of a Nipah Virus Disease Model in Hamsters, including a Comparison of Intranasal and Intraperitoneal Routes of Challenge. Pathogens 2023, 12, 976. https://doi.org/10.3390/pathogens12080976.
- 91.
Geisbert, T.W.; Feldmann, H.; Broder, C.C. Animal challenge models of henipavirus infection and pathogenesis. Henipavirus Ecol. Mol. Virol. Pathog. 2012, 359, 153–177. https://doi.org/10.1007/82_2012_208.
- 92.
Diederich, S.; Babiuk, S.; Boshra, H. A Survey of Henipavirus Tropism-Our Current Understanding from a Species/Organ and Cellular Level. Viruses 2023, 15, 2048. https://doi.org/10.3390/v15102048.
- 93.
de Wit, E.; Munster, V.J. Animal models of disease shed light on Nipah virus pathogenesis and transmission. J. Pathol. 2015, 235, 196–205. https://doi.org/10.1002/path.4444.
- 94.
Satterfield, B.A.; Cross, R.W.; Fenton, K.A.; Borisevich, V.; Agans, K.N.; Deer, D.J.; Graber, J.; Basler, C.F.; Geisbert, T.W.; Mire, C.E. Nipah Virus C and W Proteins Contribute to Respiratory Disease in Ferrets. J. Virol. 2016, 90, 6326–6343. https://doi.org/10.1128/jvi.00215-16.

This work is licensed under a Creative Commons Attribution 4.0 International License.


