- Open Access
- Review
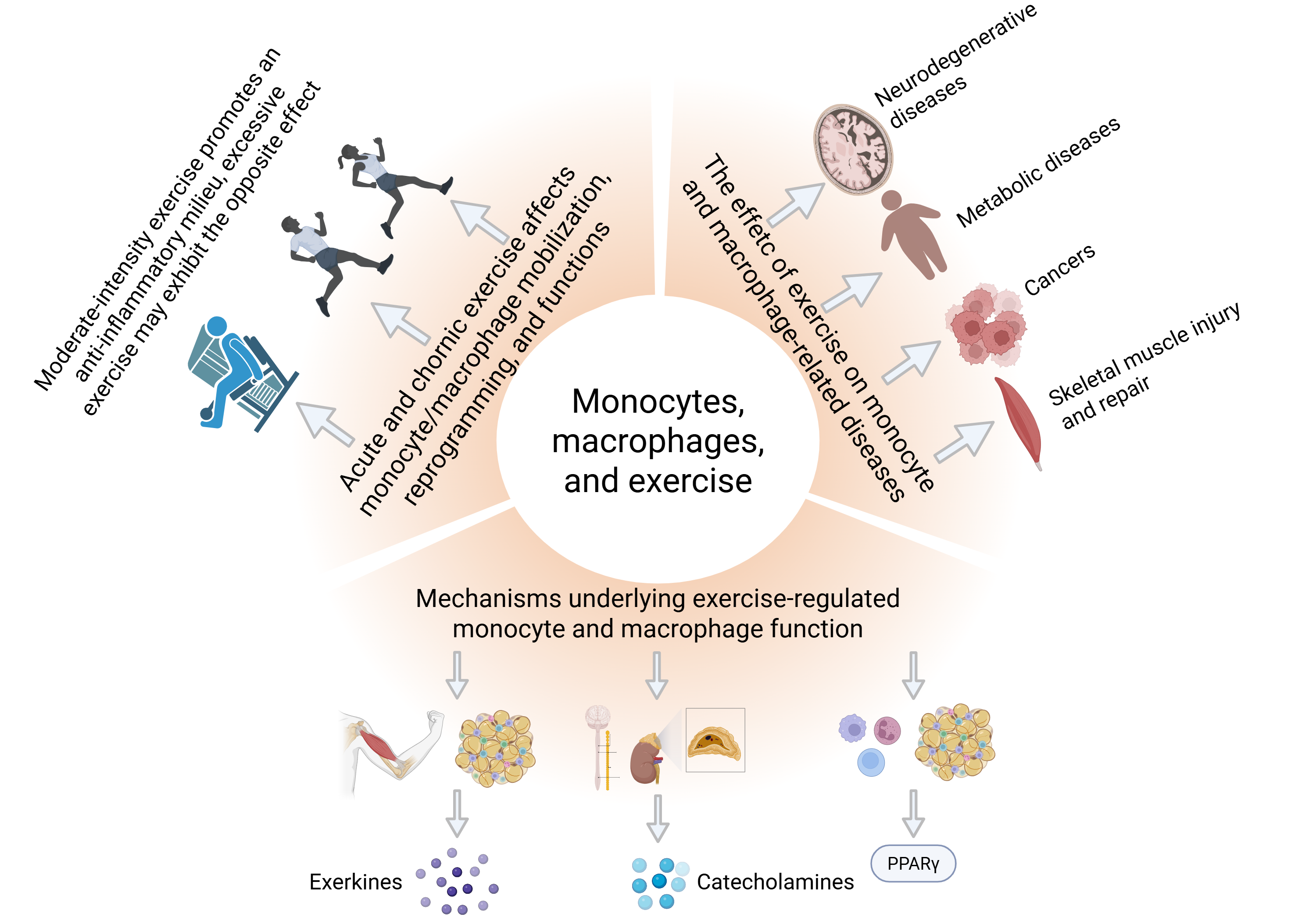
Monocytes, Macrophages, and Exercise Prescription: A New Frontier in Immunomodulation
- Tan Zhang 1,*,
- Li-Hao Huang 2,*
Author Information
Received: 06 Aug 2025 | Revised: 03 Dec 2025 | Accepted: 08 Dec 2025 | Published: 04 Feb 2026
Abstract
Graphical Abstract
Keywords
exercise | monocyte | macrophage | inflammation | phagocytosis | exerkines | PPARs
References
- 1.
Ostman, C.; Smart, N.A.; Morcos, D.; Duller, A.; Ridley, W.; Jewiss, D. The effect of exercise training on clinical outcomes in patients with the metabolic syndrome: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2017, 16, 110. https://doi.org/10.1186/s12933-017-0590-y.
- 2.
He, A.; Pu, Y.; Jia, C.; Wu, M.; He, H.; Xia, Y. The influence of exercise on cancer risk, the tumor microenvironment and the treatment of cancer. Sports Med. 2024, 54, 1371–1397. https://doi.org/10.1007/s40279-024-02031-2.
- 3.
Wang, Q.; Zhou, W. Roles and molecular mechanisms of physical exercise in cancer prevention and treatment. J. Sport Health Sci. 2021, 10, 201–210. https://doi.org/10.1016/j.jshs.2020.07.008.
- 4.
Courneya, K.S.; Vardy, J.L.; O’Callaghan, C.J.; Gill, S.; Friedenreich, C.M.; Wong, R.; Dhillon, H.M.; Coyle, V.; Chua, N.S.; Jonker, D.J.; et al. Structured Exercise after Adjuvant Chemotherapy for Colon Cancer. N. Engl. J. Med. 2025, 393, 13–25. https://doi.org/10.1056/NEJMoa2502760.
- 5.
Zhao, R. Can exercise benefits be harnessed with drugs? A new way to combat neurodegenerative diseases by boosting neurogenesis. Transl. Neurodegener. 2024, 13, 36. https://doi.org/10.1186/s40035-024-00428-7.
- 6.
Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. https://doi.org/10.1016/j.jshs.2018.09.009.
- 7.
Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. https://doi.org/10.1038/nri3041.
- 8.
Proschinger, S.; Winker, M.; Joisten, N.; Bloch, W.; Palmowski, J.; Zimmer, P. The effect of exercise on regulatory T cells: A systematic review of human and animal studies with future perspectives and methodological recommendations. Exerc. Immunol. Rev. 2021, 27, 142–166.
- 9.
Walzik, D.; Belen, S.; Wilisch, K.; Kupjetz, M.; Kirschke, S.; Esser, T.; Joisten, N.; Schenk, A.; Proschinger, S.; Zimmer, P. Impact of exercise on markers of B cell-related immunity: A systematic review. J. Sport Health Sci. 2024, 13, 339–352. https://doi.org/10.1016/j.jshs.2023.10.002.
- 10.
Langston, P.K.; Sun, Y.; Ryback, B.A.; Mueller, A.L.; Spiegelman, B.M.; Benoist, C.; Mathis, D. Regulatory T cells shield muscle mitochondria from interferon-gamma-mediated damage to promote the beneficial effects of exercise. Sci. Immunol. 2023, 8, i5377. https://doi.org/10.1126/sciimmunol.adi5377.
- 11.
Guan, F.; Wang, R.; Yi, Z.; Luo, P.; Liu, W.; Xie, Y.; Liu, Z.; Xia, Z.; Zhang, H.; Cheng, Q.; Tissue macrophages: Origin; heterogenity; biological functions; diseases; therapeutic targets. Signal Transduct. Target Ther. 2025, 10, 93. https://doi.org/10.1038/s41392-025-02124-y.
- 12.
Bian, Z.; Gong, Y.; Huang, T.; Lee, C.; Bian, L.; Bai, Z.; Shi, H.; Zeng, Y.; Liu, C.; He, J.; et al. Deciphering human macrophage development at single-cell resolution. Nature 2020, 582, 571–576. https://doi.org/10.1038/s41586-020-2316-7.
- 13.
Park, M.D.; Silvin, A.; Ginhoux, F.; Merad, M. Macrophages in health and disease. Cell 2022, 185, 4259–4279. https://doi.org/10.1016/j.cell.2022.10.007.
- 14.
Meyer, T.; Lucia, A.; Earnest, C.P.; Kindermann, W. A conceptual framework for performance diagnosis and training prescription from submaximal gas exchange parameters—Theory and application. Int. J. Sports Med. 2005, 26, S38-S48. https://doi.org/10.1055/s-2004-830514.
- 15.
Jamnick, N.A.; Pettitt, R.W.; Granata, C.; Pyne, D.B.; Bishop, D.J. An examination and critique of current methods to determine exercise intensity. Sports Med. 2020, 50, 1729–1756. https://doi.org/10.1007/s40279-020-01322-8.
- 16.
Guo, S.; Huang, Y.; Zhang, Y.; Huang, H.; Hong, S.; Liu, T. Impacts of exercise interventions on different diseases and organ functions in mice. J. Sport Health Sci. 2020, 9, 53–73. https://doi.org/10.1016/j.jshs.2019.07.004.
- 17.
van der Geest, K.; Wang, Q.; Eijsvogels, T.; Koenen, H.; Joosten, I.; Brouwer, E.; Hopman, M.; Jacobs, J.; Boots, A. Changes in peripheral immune cell numbers and functions in octogenarian walkers—An acute exercise study. Immun. Ageing 2017, 14, 5. https://doi.org/10.1186/s12979-017-0087-2.
- 18.
Koivula, T.; Lempiainen, S.; Rinne, P.; Rannikko, J.H.; Hollmen, M.; Sundberg, C.J.; Rundqvist, H.; Minn, H.; Heinonen, I. The effect of acute exercise on circulating immune cells in newly diagnosed breast cancer patients. Sci. Rep. 2023, 13, 6561. https://doi.org/10.1038/s41598-023-33432-4.
- 19.
Simpson, R.J.; Mcfarlin, B.K.; Mcsporran, C.; Spielmann, G.; Hartaigh, B.; Guy, K. Toll-like receptor expression on classic and pro-inflammatory blood monocytes after acute exercise in humans. Brain Behav. Immun. 2009, 23, 232–239. https://doi.org/10.1016/j.bbi.2008.09.013.
- 20.
Kaufmann, C.C.; Wegberger, C.; Tscharre, M.; Haller, P.M.; Piackova, E.; Vujasin, I.; Kassem, M.; Tentzeris, I.; Freynhofer, M.K.; Jager, B.; et al. Effect of marathon and ultra-marathon on inflammation and iron homeostasis. Scand. J. Med. Sci. Sports 2021, 31, 542–552. https://doi.org/10.1111/sms.13869.
- 21.
Rooney, B.V.; Bigley, A.B.; Lavoy, E.C.; Laughlin, M.; Pedlar, C.; Simpson, R.J. Lymphocytes and monocytes egress peripheral blood within minutes after cessation of steady state exercise: A detailed temporal analysis of leukocyte extravasation. Physiol. Behav. 2018, 194, 260–267. https://doi.org/10.1016/j.physbeh.2018.06.008.
- 22.
Tavares-Silva, E.; Leite, G.; Batatinha, H.; Resende, A.; Lemos, V.A.; Marques, C.G.; Lancha-Jr, A.H.; Rosa, N.J.; Thomatieli-Santos, R. Thirty days of double-strain probiotic supplementation increases monocyte phagocytosis in marathon runners. Br. J. Nutr. 2024, 132, 298–308. https://doi.org/10.1017/S0007114524001259.
- 23.
Bay, M.L.; Heywood, S.; Wedell-Neergaard, A.S.; Schauer, T.; Lehrskov, L.L.; Christensen, R.H.; Legard, G.E.; Jensen, P.O.; Krogh-Madsen, R.; Ellingsgaard, H. Human immune cell mobilization during exercise: Effect of IL-6 receptor blockade. Exp. Physiol. 2020, 105, 2086–2098. https://doi.org/10.1113/EP088864.
- 24.
Scharhag, J.; Meyer, T.; Gabriel, H.H.; Schlick, B.; Faude, O.; Kindermann, W. Does prolonged cycling of moderate intensity affect immune cell function? Br. J. Sports Med. 2005, 39, 171–177. https://doi.org/10.1136/bjsm.2004.013060.
- 25.
Hong, S.; Mills, P.J. Effects of an exercise challenge on mobilization and surface marker expression of monocyte subsets in individuals with normal vs. Elevated blood pressure. Brain Behav. Immun. 2008, 22, 590–599. https://doi.org/10.1016/j.bbi.2007.12.003.
- 26.
Oliveira, M.; Gleeson, M. The influence of prolonged cycling on monocyte Toll-like receptor 2 and 4 expression in healthy men. Eur. J. Appl. Physiol. 2010, 109, 251–257. https://doi.org/10.1007/s00421-009-1350-9.
- 27.
Booth, S.; Florida-James, G.D.; Mcfarlin, B.K.; Spielmann, G.; O’Connor, D.P.; Simpson, R.J. The impact of acute strenuous exercise on TLR2, TLR4 and HLA.DR expression on human blood monocytes induced by autologous serum. Eur. J. Appl. Physiol. 2010, 110, 1259–1268. https://doi.org/10.1007/s00421-010-1616-2.
- 28.
de Matos, M.A.; Duarte, T.C.; Ottone, V.O.; Sampaio, P.F.; Costa, K.B.; de Oliveira, M.F.; Moseley, P.L.; Schneider, S.M.; Coimbra, C.C.; Brito-Melo, G.E.; et al. The effect of insulin resistance and exercise on the percentage of CD16(+) monocyte subset in obese individuals. Cell Biochem. Funct. 2016, 34, 209–216. https://doi.org/10.1002/cbf.3178.
- 29.
Stromberg, A.; Rullman, E.; Jansson, E.; Gustafsson, T. Exercise-induced upregulation of endothelial adhesion molecules in human skeletal muscle and number of circulating cells with remodeling properties. J. Appl. Physiol. 2017, 122, 1145–1154. https://doi.org/10.1152/japplphysiol.00956.2016.
- 30.
Radom-Aizik, S.; Zaldivar, F.J.; Haddad, F.; Cooper, D.M. Impact of brief exercise on circulating monocyte gene and microRNA expression: Implications for atherosclerotic vascular disease. Brain Behav. Immun. 2014, 39, 121–129. https://doi.org/10.1016/j.bbi.2014.01.003.
- 31.
Dimitrov, S.; Shaikh, F.; Pruitt, C.; Green, M.; Wilson, K.; Beg, N.; Hong, S. Differential TNF production by monocyte subsets under physical stress: Blunted mobilization of proinflammatory monocytes in prehypertensive individuals. Brain Behav. Immun. 2013, 27, 101–108. https://doi.org/10.1016/j.bbi.2012.10.003.
- 32.
Euteneuer, F.; Schwarz, M.J.; Schmidmaier, R.; Hennings, A.; Riemer, S.; Stapf, T.M.; Selberdinger, V.B.; Sussenbach, P.; Dannehl, K.; Rief, W. Blunted exercise-induced mobilization of monocytes in somatization syndromes and major depression. J. Affect Disord. 2014, 166, 156–164. https://doi.org/10.1016/j.jad.2014.04.060.
- 33.
Kawazu, T.; Nakamura, T.; Moriki, T.; Kamijo, Y.I.; Nishimura, Y.; Kinoshita, T.; Tajima, F. Aerobic exercise combined with noninvasive positive pressure ventilation increases serum Brain-Derived neurotrophic factor in healthy males. PM&R 2016, 8, 1136–1141. https://doi.org/10.1016/j.pmrj.2016.05.004.
- 34.
Viana, J.L.; Kosmadakis, G.C.; Watson, E.L.; Bevington, A.; Feehally, J.; Bishop, N.C.; Smith, A.C. Evidence for anti-inflammatory effects of exercise in CKD. J. Am. Soc. Nephrol. 2014, 25, 2121–2130. https://doi.org/10.1681/ASN.2013070702.
- 35.
Gillum, T.; Kuennen, M.; Mckenna, Z.; Castillo, M.; Jordan-Patterson, A.; Bohnert, C. Exercise does not increase salivary lymphocytes, monocytes, or granulocytes, but does increase salivary lysozyme. J. Sports Sci. 2017, 35, 1294–1299. https://doi.org/10.1080/02640414.2016.1221522.
- 36.
Heimbeck, I.; Hofer, T.P.; Eder, C.; Wright, A.K.; Frankenberger, M.; Marei, A.; Boghdadi, G.; Scherberich, J.; Ziegler-Heitbrock, L. Standardized single-platform assay for human monocyte subpopulations: Lower CD14+CD16++ monocytes in females. Cytometry A 2010, 77, 823–830. https://doi.org/10.1002/cyto.a.20942.
- 37.
Wonner, R.; Wallner, S.; Orso, E.; Schmitz, G. Effects of acute exercise on monocyte subpopulations in metabolic syndrome patients. Cytom. B Clin. Cytom. 2018, 94, 596–605. https://doi.org/10.1002/cyto.b.21387.
- 38.
Steppich, B.; Dayyani, F.; Gruber, R.; Lorenz, R.; Mack, M.; Ziegler-Heitbrock, H.W. Selective mobilization of CD14+CD16+ monocytes by exercise. Am. J. Physiol. Cell Physiol. 2000, 279, C578–C586. https://doi.org/10.1152/ajpcell.2000.279.3.C578.
- 39.
Slusher, A.L.; Zuniga, T.M.; Acevedo, E.O. Maximal exercise alters the inflammatory phenotype and response of mononuclear cells. Med. Sci. Sports Exerc. 2018, 50, 675–683. https://doi.org/10.1249/MSS.0000000000001480.
- 40.
Van Craenenbroeck, A.H.; Van Ackeren, K.; Hoymans, V.Y.; Roeykens, J.; Verpooten, G.A.; Vrints, C.J.; Couttenye, M.M.; Van Craenenbroeck, E.M. Acute exercise-induced response of monocyte subtypes in chronic heart and renal failure. Mediat. Inflamm. 2014, 2014, 216534. https://doi.org/10.1155/2014/216534.
- 41.
Shantsila, E.; Tapp, L.D.; Wrigley, B.J.; Montoro-Garcia, S.; Ghattas, A.; Jaipersad, A.; Lip, G.Y. The effects of exercise and diurnal variation on monocyte subsets and monocyte-platelet aggregates. Eur. J. Clin. Investig. 2012, 42, 832–839. https://doi.org/10.1111/j.1365-2362.2012.02656.x.
- 42.
Schauer, T.; Djurhuus, S.S.; Simonsen, C.; Brasso, K.; Christensen, J.F. The effects of acute exercise and inflammation on immune function in early-stage prostate cancer. Brain Behav. Immun. Health 2022, 25, 100508. https://doi.org/10.1016/j.bbih.2022.100508.
- 43.
Leicht, C.A.; Paulson, T.A.; Goosey-Tolfrey, V.L.; Bishop, N.C. Arm and Intensity-Matched leg exercise induce similar inflammatory responses. Med. Sci. Sports Exerc. 2016, 48, 1161–1168. https://doi.org/10.1249/MSS.0000000000000874.
- 44.
Guereschi, M.G.; Prestes, J.; Donatto, F.F.; Dias, R.; Frollini, A.B.; Ferreira, C.K.; Cavaglieri, C.R.; Palanch, A.C. Exercise induced alterations in rat monocyte number, morphology, and function. Int. J. Exerc. Sci. 2008, 1, 71–78. https://doi.org/10.70252/JNVX6872.
- 45.
Wang, J.S.; Lee, T.; Chow, S.E. Role of exercise intensities in oxidized low-density lipoprotein-mediated redox status of monocyte in men. J. Appl. Physiol. 2006, 101, 740–744. https://doi.org/10.1152/japplphysiol.00144.2006.
- 46.
O’Carroll, L.; Wardrop, B.; Murphy, R.P.; Ross, M.D.; Harrison, M. Circulating angiogenic cell response to sprint interval and continuous exercise. Eur. J. Appl. Physiol. 2019, 119, 743–752. https://doi.org/10.1007/s00421-018-04065-7.
- 47.
Durrer, C.; Francois, M.; Neudorf, H.; Little, J.P. Acute high-intensity interval exercise reduces human monocyte Toll-like receptor 2 expression in type 2 diabetes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R529–R538. https://doi.org/10.1152/ajpregu.00348.2016.
- 48.
Ramel, A.; Wagner, K.H.; Elmadfa, I. Acute impact of submaximal resistance exercise on immunological and hormonal parameters in young men. J. Sports Sci. 2003, 21, 1001–1008. https://doi.org/10.1080/02640410310001641395.
- 49.
Allsopp, G.L.; Addinsall, A.B.; Stephenson, G.; Basheer, F.; Gatta, P.; May, H.S.; Russell, A.P.; Wright, C.R. The acute leukocyte and cytokine response of older adults to resistance exercise in normobaric hypoxia. Biol. Sport 2023, 40, 425–438. https://doi.org/10.5114/biolsport.2023.116005.
- 50.
Wells, A.J.; Hoffman, J.R.; Jajtner, A.R.; Varanoske, A.N.; Church, D.D.; Gonzalez, A.M.; Townsend, J.R.; Boone, C.H.; Baker, K.M.; Beyer, K.S.; et al. The effect of Post-Resistance exercise amino acids on plasma MCP-1 and CCR2 expression. Nutrients 2016, 8, 409. https://doi.org/10.3390/nu8070409.
- 51.
Wiecek, M.; Maciejczyk, M.; Szymura, J.; Szygula, Z. Sex differences in oxidative stress after eccentric and concentric exercise. Redox Rep. 2017, 22, 478–485. https://doi.org/10.1080/13510002.2017.1304195.
- 52.
Paulsen, G.; Benestad, H.B.; Strom-Gundersen, I.; Morkrid, L.; Lappegard, K.T.; Raastad, T. Delayed leukocytosis and cytokine response to high-force eccentric exercise. Med. Sci. Sports Exerc. 2005, 37, 1877–1883. https://doi.org/10.1249/01.mss.0000177064.65927.98.
- 53.
Sorichter, S.; Martin, M.; Julius, P.; Schwirtz, A.; Huonker, M.; Luttmann, W.; Walterspacher, S.; Berg, A. Effects of unaccustomed and accustomed exercise on the immune response in runners. Med. Sci. Sports Exerc. 2006, 38, 1739–1745. https://doi.org/10.1249/01.mss.0000230213.62743.fb.
- 54.
Sakelliou, A.; Fatouros, I.G.; Athanailidis, I.; Tsoukas, D.; Chatzinikolaou, A.; Draganidis, D.; Jamurtas, A.Z.; Liacos, C.; Papassotiriou, I.; Mandalidis, D.; et al. Evidence of a Redox-Dependent regulation of immune responses to Exercise-Induced inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 2840643. https://doi.org/10.1155/2016/2840643.
- 55.
Cardoso, A.M.; Bagatini, M.D.; Roth, M.A.; Martins, C.C.; Rezer, J.F.; Mello, F.F.; Lopes, L.F.; Morsch, V.M.; Schetinger, M.R. Acute effects of resistance exercise and intermittent intense aerobic exercise on blood cell count and oxidative stress in trained middle-aged women. Braz. J. Med. Biol. Res. 2012, 45, 1172–1182. https://doi.org/10.1590/s0100-879x2012007500166.
- 56.
Dergaa, I.; Ben, S.H.; Romdhani, M.; Souissi, A.; Fessi, M.S.; Yousfi, N.; Masmoudi, T.; Souissi, N.; Ammar, A.; Hammouda, O. Biological responses to Short-Term maximal exercise in male police officers. Am. J. Mens. Health 2021, 15, 1013950616. https://doi.org/10.1177/15579883211040920.
- 57.
Pillon, N.J.; Smith, J.; Alm, P.S.; Chibalin, A.V.; Alhusen, J.; Arner, E.; Carninci, P.; Fritz, T.; Otten, J.; Olsson, T.; et al. Distinctive exercise-induced inflammatory response and exerkine induction in skeletal muscle of people with type 2 diabetes. Sci. Adv. 2022, 8, o3192. https://doi.org/10.1126/sciadv.abo3192.
- 58.
Wu, J.; Cheng, I.S.; Saovieng, S.; Jean, W.H.; Kao, C.L.; Liu, Y.Y.; Huang, C.Y.; Lee, T.; Ivy, J.L.; Kuo, C.H. Aerobic exercise induces tumor suppressor p16(INK4a) expression of endothelial progenitor cells in human skeletal muscle. Aging 2020, 12, 20226–20234. https://doi.org/10.18632/aging.103763.
- 59.
Bortolon, J.R.; Silva, J.A.; Murata, G.M.; Newsholme, P.; Curi, R.; Pithon-Curi, T.C.; Hatanaka, E. Persistence of inflammatory response to intense exercise in diabetic rats. Exp. Diabetes Res. 2012, 2012, 213986. https://doi.org/10.1155/2012/213986.
- 60.
Komori, T.; Morikawa, Y. Essential roles of the cytokine oncostatin M in crosstalk between muscle fibers and immune cells in skeletal muscle after aerobic exercise. J. Biol. Chem. 2022, 298, 102686. https://doi.org/10.1016/j.jbc.2022.102686.
- 61.
Kawanishi, N.; Mizokami, T.; Niihara, H.; Yada, K.; Suzuki, K. Neutrophil Depletion Attenuates Muscle Injury after Exhaustive Exercise. Med. Sci. Sports Exerc. 2016, 48, 1917–1924. https://doi.org/10.1249/MSS.0000000000000980.
- 62.
Oliveira, A.G.; Araujo, T.G.; Carvalho, B.M.; Guadagnini, D.; Rocha, G.Z.; Bagarolli, R.A.; Carvalheira, J.B.; Saad, M.J. Acute exercise induces a phenotypic switch in adipose tissue macrophage polarization in diet-induced obese rats. Obesity 2013, 21, 2545–2556. https://doi.org/10.1002/oby.20402.
- 63.
Macpherson, R.E.; Huber, J.S.; Frendo-Cumbo, S.; Simpson, J.A.; Wright, D.C. Adipose tissue insulin action and IL-6 signaling after exercise in obese mice. Med. Sci. Sports Exerc. 2015, 47, 2034–2042. https://doi.org/10.1249/MSS.0000000000000660.
- 64.
Baek, K.W.; Kim, J.H.; Yu, H.S.; Kim, J.S. Adipose Tissue Macrophage Polarization is Altered during Recovery after Exercise: A Large-Scale Flow Cytometric Study. Curr. Issues Mol. Biol. 2024, 46, 1308–1317. https://doi.org/10.3390/cimb46020083.
- 65.
Ludzki, A.C.; Krueger, E.M.; Baldwin, T.C.; Schleh, M.W.; Porsche, C.E.; Ryan, B.J.; Muir, L.A.; Singer, K.; Lumeng, C.N.; Horowitz, J.F. Acute aerobic exercise remodels the adipose tissue progenitor cell phenotype in obese adults. Front. Physiol. 2020, 11, 903. https://doi.org/10.3389/fphys.2020.00903.
- 66.
Mizokami, T.; Shimada, M.; Suzuki, K. Macrophage depletion attenuates acute renal damage after exhaustive exercise in mice. Int. J. Sports Med. 2022, 43, 964–970. https://doi.org/10.1055/a-1827-3261.
- 67.
Peake, J.M.; Roberts, L.A.; Figueiredo, V.C.; Egner, I.; Krog, S.; Aas, S.N.; Suzuki, K.; Markworth, J.F.; Coombes, J.S.; Cameron-Smith, D.; et al. The effects of cold water immersion and active recovery on inflammation and cell stress responses in human skeletal muscle after resistance exercise. J. Physiol. 2017, 595, 695–711. https://doi.org/10.1113/JP272881.
- 68.
Walton, R.G.; Kosmac, K.; Mula, J.; Fry, C.S.; Peck, B.D.; Groshong, J.S.; Finlin, B.S.; Zhu, B.; Kern, P.A.; Peterson, C.A. Human skeletal muscle macrophages increase following cycle training and are associated with adaptations that may facilitate growth. Sci. Rep. 2019, 9, 969. https://doi.org/10.1038/s41598-018-37187-1.
- 69.
Lavin, K.M.; Perkins, R.K.; Jemiolo, B.; Raue, U.; Trappe, S.W.; Trappe, T.A. Effects of aging and lifelong aerobic exercise on basal and exercise-induced inflammation in women. J. Appl. Physiol. 2020, 129, 1493–1504. https://doi.org/10.1152/japplphysiol.00655.2020.
- 70.
Przybyla, B.; Gurley, C.; Harvey, J.F.; Bearden, E.; Kortebein, P.; Evans, W.J.; Sullivan, D.H.; Peterson, C.A.; Dennis, R.A. Aging alters macrophage properties in human skeletal muscle both at rest and in response to acute resistance exercise. Exp. Gerontol. 2006, 41, 320–327. https://doi.org/10.1016/j.exger.2005.12.007.
- 71.
Jensen, S.M.; Bechshoft, C.; Heisterberg, M.F.; Schjerling, P.; Andersen, J.L.; Kjaer, M.; Mackey, A.L. Macrophage subpopulations and the acute inflammatory response of elderly human skeletal muscle to physiological resistance exercise. Front. Physiol. 2020, 11, 811. https://doi.org/10.3389/fphys.2020.00811.
- 72.
Menon, M.K.; Houchen, L.; Singh, S.J.; Morgan, M.D.; Bradding, P.; Steiner, M.C. Inflammatory and satellite cells in the quadriceps of patients with COPD and response to resistance training. Chest 2012, 142, 1134–1142. https://doi.org/10.1378/chest.11-2144.
- 73.
Zuo, Q.; Wang, S.C.; Yu, X.K.; Chao, W.W. Response of macrophages in rat skeletal muscle after eccentric exercise. Chin. J. Traumatol. 2018, 21, 88–95. https://doi.org/10.1016/j.cjtee.2017.12.001.
- 74.
Huang, S.C.; Wu, J.F.; Saovieng, S.; Chien, W.H.; Hsu, M.F.; Li, X.F.; Lee, S.D.; Huang, C.Y.; Huang, C.Y.; Kuo, C.H. Doxorubicin inhibits muscle inflammation after eccentric exercise. J. Cachexia Sarcopenia Muscle 2017, 8, 277–284. https://doi.org/10.1002/jcsm.12148.
- 75.
Minari, A.; Avila, F.; Oyama, L.M.; Thomatieli-Santos, R.V. Skeletal muscles induce recruitment of Ly6C+ macrophage subtypes and release inflammatory cytokines 3 days after downhill exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R597–R605. https://doi.org/10.1152/ajpregu.00163.2019.
- 76.
Minari, A.L.; Oyama, L.M.; Dos, S.R. Downhill exercise-induced changes in gene expression related with macrophage polarization and myogenic cells in the triceps long head of rats. Inflammation 2015, 38, 209–217. https://doi.org/10.1007/s10753-014-0024-x.
- 77.
Silveira, E.M.; Rodrigues, M.F.; Krause, M.S.; Vianna, D.R.; Almeida, B.S.; Rossato, J.S.; Oliveira, L.J.; Curi, R.; de Bittencourt, P.J. Acute exercise stimulates macrophage function: Possible role of NF-kappaB pathways. Cell Biochem. Funct. 2007, 25, 63–73. https://doi.org/10.1002/cbf.1365.
- 78.
Da, S.R.J.; Krause, M.; Fernandes, A.J.; Fernandes, J.R.; Seibt, I.L.; Rech, A.; Homem, D.B.P.J. Role of alpha- and beta-adrenoreceptors in rat monocyte/macrophage function at rest and acute exercise. J. Physiol. Biochem. 2014, 70, 363–374. https://doi.org/10.1007/s13105-013-0310-3.
- 79.
Scholer, C.M.; Marques, C.V.; Da, S.G.; Heck, T.G.; de Oliveira, J.L.; Homem, D.B.P.J. Modulation of rat monocyte/macrophage innate functions by increasing intensities of swimming exercise is associated with heat shock protein status. Mol. Cell. Biochem. 2016, 421, 111–125. https://doi.org/10.1007/s11010-016-2791-1.
- 80.
Ferreira, C.K.; Prestes, J.; Donatto, F.F.; Verlengia, R.; Navalta, J.W.; Cavaglieri, C.R. Phagocytic responses of peritoneal macrophages and neutrophils are different in rats following prolonged exercise. Clinics 2010, 65, 1167–1173. https://doi.org/10.1590/s1807-59322010001100020.
- 81.
Bote, M.E.; Garcia, J.J.; Hinchado, M.D.; Ortega, E. Fibromyalgia: Anti-inflammatory and stress responses after acute moderate exercise. PLoS ONE 2013, 8, e74524. https://doi.org/10.1371/journal.pone.0074524.
- 82.
Dimitrov, S.; Hulteng, E.; Hong, S. Inflammation and exercise: Inhibition of monocytic intracellular TNF production by acute exercise via β2-adrenergic activation. Brain Behav. Immun. 2017, 61, 60–68. https://doi.org/10.1016/j.bbi.2016.12.017.
- 83.
Toumpanakis, D.; Karatza, M.H.; Katsaounou, P.; Roussos, C.; Zakynthinos, S.; Papapetropoulos, A.; Vassilakopoulos, T. Antioxidant supplementation alters cytokine production from monocytes. J. Interferon Cytokine Res. 2009, 29, 741–748. https://doi.org/10.1089/jir.2008.0114.
- 84.
van de Vyver, M.; Engelbrecht, L.; Smith, C.; Myburgh, K.H. Neutrophil and monocyte responses to downhill running: Intracellular contents of MPO, IL-6, IL-10, pstat3, and SOCS3. Scand. J. Med. Sci. Sports 2016, 26, 638–647. https://doi.org/10.1111/sms.12497.
- 85.
Starkie, R.L.; Angus, D.J.; Rolland, J.; Hargreaves, M.; Febbraio, M.A.; Effect of prolonged, submaximal exercise and carbohydrate ingestion on monocyte intracellular cytokine production in humans. J. Physiol. 2000, 528, 647–655. https://doi.org/10.1111/j.1469-7793.2000.t01-1-00647.x.
- 86.
Martin-Cordero, L.; Garcia, J.J.; Hinchado, M.D.; Bote, E.; Manso, R.; Ortega, E. Habitual physical exercise improves macrophage IL-6 and TNF-alpha deregulated release in the obese zucker rat model of the metabolic syndrome. Neuroimmunomodulation 2011, 18, 123–130. https://doi.org/10.1159/000322053.
- 87.
Martin-Cordero, L.; Garcia, J.J.; Giraldo, E.; De la Fuente, M.; Manso, R.; Ortega, E. Influence of exercise on the circulating levels and macrophage production of IL-1beta and IFNgamma affected by metabolic syndrome: An obese Zucker rat experimental animal model. Eur. J. Appl. Physiol. 2009, 107, 535–543. https://doi.org/10.1007/s00421-009-1140-4.
- 88.
Roberts, M.J.; Hamrouni, M.; Linsley, V.; Moorthy, A.; Bishop, N.C. Exercise as an anti-inflammatory Therapy in Axial Spondyloarthritis Therapeutic Intervention (EXTASI) study: A randomized controlled trial. Rheumatol. Adv. Pract. 2024, 8, e62. https://doi.org/10.1093/rap/rkae062.
- 89.
Mazur, M.; Glodzik, J.; Szczepaniak, P.; Nosalski, R.; Siedlinski, M.; Skiba, D.; Rewiuk, K.; Salakowski, A.; Czesnikiewicz-Guzik, M.; Grodzicki, T.; et al. Effects of controlled physical activity on immune cell phenotype in peripheral blood in prehypertension—Studies in preclinical model and randomised crossover study. J. Physiol. Pharmacol. 2018, 69, 875–887. https://doi.org/10.26402/jpp.2018.6.12.
- 90.
Tenorio, T.; Balagopal, P.B.; Andersen, L.B.; Ritti-Dias, R.M.; Hill, J.O.; Lofrano-Prado, M.C.; Prado, W.L. Effect of low- versus High-Intensity exercise training on biomarkers of inflammation and endothelial dysfunction in adolescents with obesity: A 6-Month randomized exercise intervention study. Pediatr. Exerc. Sci. 2018, 30, 96–105. https://doi.org/10.1123/pes.2017-0067.
- 91.
Ruffino, J.S.; Davies, N.A.; Morris, K.; Ludgate, M.; Zhang, L.; Webb, R.; Thomas, A.W. Moderate-intensity exercise alters markers of alternative activation in circulating monocytes in females: A putative role for PPARgamma. Eur. J. Appl. Physiol. 2016, 116, 1671–1682. https://doi.org/10.1007/s00421-016-3414-y.
- 92.
Michishita, R.; Shono, N.; Inoue, T.; Tsuruta, T.; Node, K. Effect of exercise therapy on monocyte and neutrophil counts in overweight women. Am. J. Med. Sci. 2010, 339, 152–156. https://doi.org/10.1097/MAJ.0b013e3181c6a980.
- 93.
Carpenter, K.C.; Strohacker, K.; Breslin, W.L.; Lowder, T.W.; Agha, N.H.; Mcfarlin, B.K. Effects of exercise on weight loss and monocytes in obese mice. Comp. Med. 2012, 62, 21–26.
- 94.
Huang, S.C.; Wong, M.K.; Lin, P.J.; Tsai, F.C.; Chu, J.J.; Wu, M.Y.; Fu, T.C.; Wang, J.S. Short-term intensive training attenuates the exercise-induced interaction of mono-1/2 cells and platelets after coronary bypass in cardiac patients. Thromb. Haemost. 2017, 117, 1761–1771. https://doi.org/10.1160/TH17-03-0184.
- 95.
Ruiz-Iglesias, P.; Estruel-Amades, S.; Camps-Bossacoma, M.; Massot-Cladera, M.; Franch, A.; Perez-Cano, F.J.; Castell, M. Influence of hesperidin on systemic immunity of rats following an intensive training and exhausting exercise. Nutrients 2020, 12, 1291. https://doi.org/10.3390/nu12051291.
- 96.
Markofski, M.M.; Flynn, M.G.; Carrillo, A.E.; Armstrong, C.L.; Campbell, W.W.; Sedlock, D.A. Resistance exercise training-induced decrease in circulating inflammatory CD14+CD16+ monocyte percentage without weight loss in older adults. Eur. J. Appl. Physiol. 2014, 114, 1737–1748. https://doi.org/10.1007/s00421-014-2902-1.
- 97.
Da, S.I.; Santos, M.A.; Galvao, S.L.; Dorneles, G.P.; Lira, F.S.; Romao, P.; Peres, A. Blood flow restriction impairs the inflammatory adaptations of strength training in overweight men: A clinical randomized trial. Appl. Physiol. Nutr. Metab. 2020, 45, 659–666. https://doi.org/10.1139/apnm-2019-0700.
- 98.
Spiliopoulou, P.; Rousakis, P.; Panteli, C.; Eleutherakis-Papaiakovou, E.; Migkou, M.; Kanellias, N.; Ntanasis-Stathopoulos, I.; Malandrakis, P.; Theodorakakou, F.; Fotiou, D.; et al. Effects of exercise training on the bone marrow immune microenvironment and minimal residual disease in multiple myeloma patients following First-Line treatment. Scand. J. Med. Sci. Sports 2025, 35, e70020. https://doi.org/10.1111/sms.70020.
- 99.
Timmerman, K.L.; Flynn, M.G.; Coen, P.M.; Markofski, M.M.; Pence, B.D. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: A role in the anti-inflammatory influence of exercise? J. Leukoc. Biol. 2008, 84, 1271–1278. https://doi.org/10.1189/jlb.0408244.
- 100.
Gil-Cosano, J.J.; Plaza-Florido, A.; Gracia-Marco, L.; Migueles, J.H.; Cadenas-Sanchez, C.; Olvera-Rojas, M.; Ubago-Guisado, E.; Labayen, I.; Lucia, A.; Ortega, F.B. Effects of combined aerobic and resistance training on the inflammatory profile of children with overweight/obesity: A randomized clinical trial. Pediatr. Obes. 2024, 19, e13152. https://doi.org/10.1111/ijpo.13152.
- 101.
Shimizu, K.; Suzuki, N.; Imai, T.; Aizawa, K.; Nanba, H.; Hanaoka, Y.; Kuno, S.; Mesaki, N.; Kono, I.; Akama, T. Monocyte and T-cell responses to exercise training in elderly subjects. J. Strength Cond. Res. 2011, 25, 2565–2572. https://doi.org/10.1519/JSC.0b013e3181fc5e67.
- 102.
Chamorro-Vina, C.; Ruiz, J.R.; Santana-Sosa, E.; Gonzalez, V.M.; Madero, L.; Perez, M.; Fleck, S.J.; Perez, A.; Ramirez, M.; Lucia, A. Exercise during hematopoietic stem cell transplant hospitalization in children. Med. Sci. Sports Exerc. 2010, 42, 1045–1053. https://doi.org/10.1249/MSS.0b013e3181c4dac1.
- 103.
de Matos, M.A.; Garcia, B.; Vieira, D.V.; de Oliveira, M.; Costa, K.B.; Aguiar, P.F.; Magalhaes, F.C.; Brito-Melo, G.A.; Amorim, F.T.; Rocha-Vieira, E. High-intensity interval training reduces monocyte activation in obese adults. Brain Behav. Immun. 2019, 80, 818–824. https://doi.org/10.1016/j.bbi.2019.05.030.
- 104.
Bartlett, D.B.; Willis, L.H.; Slentz, C.A.; Hoselton, A.; Kelly, L.; Huebner, J.L.; Kraus, V.B.; Moss, J.; Muehlbauer, M.J.; Spielmann, G.; et al. Ten weeks of high-intensity interval walk training is associated with reduced disease activity and improved innate immune function in older adults with rheumatoid arthritis: A pilot study. Arthritis Res. Ther. 2018, 20, 127. https://doi.org/10.1186/s13075-018-1624-x.
- 105.
Kawanishi, N.; Yano, H.; Mizokami, T.; Takahashi, M.; Oyanagi, E.; Suzuki, K. Exercise training attenuates hepatic inflammation, fibrosis and macrophage infiltration during diet induced-obesity in mice. Brain Behav. Immun. 2012, 26, 931–941. https://doi.org/10.1016/j.bbi.2012.04.006.
- 106.
Kawanishi, N.; Mizokami, T.; Yada, K.; Suzuki, K. Exercise training suppresses scavenger receptor CD36 expression in kupffer cells of nonalcoholic steatohepatitis model mice. Physiol. Rep. 2018, 6, e13902. https://doi.org/10.14814/phy2.13902.
- 107.
Sun, M.; Zhao, X.; Li, X.; Wang, C.; Lin, L.; Wang, K.; Sun, Y.; Ye, W.; Li, H.; Zhang, Y.; et al. Aerobic exercise ameliorates liver injury in Db/Db mice by attenuating oxidative stress, apoptosis and inflammation through the nrf2 and JAK2/STAT3 signalling pathways. J. Inflamm. Res. 2023, 16, 4805–4819. https://doi.org/10.2147/JIR.S426581.
- 108.
Jeong, J.H.; Lee, Y.R.; Park, H.G.; Lee, W.L. The effects of either resveratrol or exercise on macrophage infiltration and switching from M1 to M2 in high fat diet mice. J. Exerc. Nutrition Biochem. 2015, 19, 65–72. https://doi.org/10.5717/jenb.2015.15060203.
- 109.
Gehrke, N.; Biedenbach, J.; Huber, Y.; Straub, B.K.; Galle, P.R.; Simon, P.; Schattenberg, J.M. Voluntary exercise in mice fed an obesogenic diet alters the hepatic immune phenotype and improves metabolic parameters—An animal model of life style intervention in NAFLD. Sci. Rep. 2019, 9, 4007. https://doi.org/10.1038/s41598-018-38321-9.
- 110.
Huber, Y.; Gehrke, N.; Biedenbach, J.; Helmig, S.; Simon, P.; Straub, B.K.; Bergheim, I.; Huber, T.; Schuppan, D.; Galle, P.R.; et al. Voluntary distance running prevents TNF-mediated liver injury in mice through alterations of the intrahepatic immune milieu. Cell Death Dis. 2017, 8, e2893. https://doi.org/10.1038/cddis.2017.266.
- 111.
Yazdani, H.O.; Kaltenmeier, C.; Morder, K.; Moon, J.; Traczek, M.; Loughran, P.; Zamora, R.; Vodovotz, Y.; Li, F.; Wang, J.H.; et al. Exercise training decreases hepatic injury and metastases through changes in immune response to liver Ischemia/Reperfusion in mice. Hepatology 2021, 73, 2494–2509. https://doi.org/10.1002/hep.31552.
- 112.
Fredrickson, G.; Barrow, F.; Dietsche, K.; Parthiban, P.; Khan, S.; Robert, S.; Demirchian, M.; Rhoades, H.; Wang, H.; Adeyi, O.; et al. Exercise of high intensity ameliorates hepatic inflammation and the progression of NASH. Mol. Metab. 2021, 53, 101270. https://doi.org/10.1016/j.molmet.2021.101270.
- 113.
Zhang, H.; Chen, T.; Ren, J.; Xia, Y.; Onuma, A.; Wang, Y.; He, J.; Wu, J.; Wang, H.; Hamad, A.; et al. Pre-operative exercise therapy triggers anti-inflammatory trained immunity of Kupffer cells through metabolic reprogramming. Nat. Metab. 2021, 3, 843–858. https://doi.org/10.1038/s42255-021-00402-x.
- 114.
Linden, M.A.; Fletcher, J.A.; Morris, E.M.; Meers, G.M.; Laughlin, M.H.; Booth, F.W.; Sowers, J.R.; Ibdah, J.A.; Thyfault, J.P.; Rector, R.S. Treating NAFLD in OLETF rats with vigorous-intensity interval exercise training. Med. Sci. Sports Exerc. 2015, 47, 556–567. https://doi.org/10.1249/MSS.0000000000000430.
- 115.
Wang, Y.; Guo, Y.; Xu, Y.; Wang, W.; Zhuang, S.; Wang, R.; Xiao, W. HIIT ameliorates inflammation and lipid metabolism by regulating macrophage polarization and mitochondrial dynamics in the liver of type 2 diabetes mellitus mice. Metabolites 2022, 13, 14. https://doi.org/10.3390/metabo13010014.
- 116.
Lin, S.; Zhang, A.; Yuan, L.; Wang, Y.; Zhang, C.; Jiang, J.; Xu, H.; Yuan, H.; Yao, H.; Zhang, Q.; et al. Targeting parvalbumin promotes M2 macrophage polarization and energy expenditure in mice. Nat. Commun. 2022, 13, 3301. https://doi.org/10.1038/s41467-022-30757-y.
- 117.
Auerbach, P.; Nordby, P.; Bendtsen, L.Q.; Mehlsen, J.L.; Basnet, S.K.; Vestergaard, H.; Ploug, T.; Stallknecht, B. Differential effects of endurance training and weight loss on plasma adiponectin multimers and adipose tissue macrophages in younger, moderately overweight men. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R490–R498. https://doi.org/10.1152/ajpregu.00575.2012.
- 118.
Ahn, C.; Zhang, T.; Yang, G.; Rode, T.; Varshney, P.; Ghayur, S.J.; Chugh, O.K.; Jiang, H.; Horowitz, J.F. Years of endurance exercise training remodel abdominal subcutaneous adipose tissue in adults with overweight or obesity. Nat. Metab. 2024, 6, 1819–1836. https://doi.org/10.1038/s42255-024-01103-x.
- 119.
Kawanishi, N.; Mizokami, T.; Yano, H.; Suzuki, K. Exercise attenuates M1 macrophages and CD8+ T cells in the adipose tissue of obese mice. Med. Sci. Sports Exerc. 2013, 45, 1684–1693. https://doi.org/10.1249/MSS.0b013e31828ff9c6.
- 120.
Linden, M.A.; Pincu, Y.; Martin, S.A.; Woods, J.A.; Baynard, T. Moderate exercise training provides modest protection against adipose tissue inflammatory gene expression in response to high-fat feeding. Physiol. Rep. 2014, 2, e12071. https://doi.org/10.14814/phy2.12071.
- 121.
Baek, K.W.; Lee, D.I.; Kang, S.A.; Yu, H.S. Differences in macrophage polarization in the adipose tissue of obese mice under various levels of exercise intensity. J. Physiol. Biochem. 2020, 76, 159–168. https://doi.org/10.1007/s13105-020-00731-7.
- 122.
Salama, A.; Amin, M.M.; Hassan, A. Effects of oleic acid and/or exercise on diet-induced thermogenesis and obesity in rats: Involvement of beige adipocyte differentiation and macrophage M1 inhibition. Res. Pharm. Sci. 2023, 18, 219–230. https://doi.org/10.4103/1735-5362.367800.
- 123.
Shanaki, M.; Khosravi, M.; Khoshdooni-Farahani, A.; Dadashi, A.; Heydari, M.F.; Delfan, M.; Jafary, H.; Gorgani-Firuzjaee, S. High-Intensity interval training reversed High-Fat Diet-Induced M1-Macrophage polarization in rat adipose tissue via inhibition of NOTCH signaling. J. Inflamm. Res. 2020, 13, 165–174. https://doi.org/10.2147/JIR.S237049.
- 124.
Kawanishi, N.; Yano, H.; Yokogawa, Y.; Suzuki, K. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc. Immunol. Rev. 2010, 16, 105–118.
- 125.
Wang, S.S.; Gu, Q.; Liu, N.; Li, J.; Liu, X. Aerobic exercise attenuates ectopic renal sinus adipose tissue accumulation-related renal hypoxia injury in obese mice. Life Sci. 2021, 279, 119106. https://doi.org/10.1016/j.lfs.2021.119106.
- 126.
Kolahdouzi, S.; Talebi-Garakani, E.; Hamidian, G.; Safarzade, A. Exercise training prevents high-fat diet-induced adipose tissue remodeling by promoting capillary density and macrophage polarization. Life Sci. 2019, 220, 32–43. https://doi.org/10.1016/j.lfs.2019.01.037.
- 127.
Xu, X.; Ying, Z.; Cai, M.; Xu, Z.; Li, Y.; Jiang, S.Y.; Tzan, K.; Wang, A.; Parthasarathy, S.; He, G.; et al. Exercise ameliorates high-fat diet-induced metabolic and vascular dysfunction, and increases adipocyte progenitor cell population in brown adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R1115–R1125. https://doi.org/10.1152/ajpregu.00806.2010.
- 128.
Wang, J.; Polaki, V.; Chen, S.; Bihl, J.C. Exercise improves endothelial function associated with alleviated inflammation and oxidative stress of perivascular adipose tissue in type 2 diabetic mice. Oxid. Med. Cell. Longev. 2020, 2020, 8830537. https://doi.org/10.1155/2020/8830537.
- 129.
Guo, Y.; Zhang, Q.; Yang, D.; Chen, P.; Xiao, W. HIIT promotes m2 macrophage polarization and sympathetic nerve density to induce adipose tissue browning in T2DM mice. Biomolecules 2024, 14, 246. https://doi.org/10.3390/biom14030246.
- 130.
Da, C.F.C.; Da, C.R.K.; de Melo, D.G.; de Campos, T.; Dos, S.C.R.; Simabuco, F.M.; Da, S.A.; Cintra, D.E.; Ropelle, E.R.; Pauli, J.R.; et al. Short-term strength exercise reduces the macrophage M1/M2 ratio in white adipose tissue of obese animals. Life Sci. 2023, 329, 121916. https://doi.org/10.1016/j.lfs.2023.121916.
- 131.
Luo, W.; Ai, L.; Wang, B.; Wang, L.; Gan, Y.; Liu, C.; Jensen, J.; Zhou, Y. Eccentric exercise and dietary restriction inhibits M1 macrophage polarization activated by high-fat diet-induced obesity. Life Sci. 2020, 243, 117246. https://doi.org/10.1016/j.lfs.2019.117246.
- 132.
Luo, W.; Zhou, Y.; Tang, Q.; Ai, L.; Zhang, Y. Modulation of TRIB3 and macrophage phenotype to attenuate insulin resistance after downhill running in mice. Front. Physiol. 2021, 12, 637432. https://doi.org/10.3389/fphys.2021.637432.
- 133.
Sahl, R.E.; Patsi, I.; Hansen, M.T.; Romer, T.; Frandsen, J.; Rasmusen, H.K.; Ingersen, A.; Poulsen, S.S.; Dela, F.; Larsen, S.; et al. Prolonged endurance exercise increases macrophage content and mitochondrial respiration in adipose tissue in trained men. J. Clin. Endocrinol. Metab. 2024, 109, e799–e808. https://doi.org/10.1210/clinem/dgad509.
- 134.
Baek, K.W.; Lee, D.I.; Jeong, M.J.; Kang, S.A.; Choe, Y.; Yoo, J.I.; Yu, H.S.; Kim, J.S. Effects of lifelong spontaneous exercise on the M1/M2 macrophage polarization ratio and gene expression in adipose tissue of super-aged mice. Exp. Gerontol. 2020, 141, 111091. https://doi.org/10.1016/j.exger.2020.111091.
- 135.
Sahl, R.E.; Andersen, P.R.; Gronbaek, K.; Morville, T.H.; Rosenkilde, M.; Rasmusen, H.K.; Poulsen, S.S.; Prats, C.; Dela, F.; Helge, J.W. Repeated excessive exercise attenuates the Anti-Inflammatory effects of exercise in older men. Front. Physiol. 2017, 8, 407. https://doi.org/10.3389/fphys.2017.00407.
- 136.
Appleyard, C.B.; Cruz, M.L.; Velazquez-Cruz, J.; Rivera-Mendez, R.M.; Jimenez-Garcia, J.G.; Rivera, L.A.; Del, M.M.M.; Flores, I.; Al-Nakkash, L.; Chompre, G. Voluntary wheel running reduces vesicle development in an endometriosis animal model through modulation of immune parameters. Front. Reprod. Health 2021, 3, 826541. https://doi.org/10.3389/frph.2021.826541.
- 137.
Zeng, N.; Liao, T.; Chen, X.Y.; Yan, Z.P.; Li, J.T.; Ni, G.X. Treadmill running induces remodeling of the infrapatellar fat pad in an intensity-dependent manner. J. Orthop. Surg. Res. 2021, 16, 354. https://doi.org/10.1186/s13018-021-02501-7.
- 138.
Del, B.V.; Ferreira, G.; Bochi, A.; Pinto, P.R.; Rodrigues, L.G.; Furukawa, L.; Okamoto, M.M.; Almeida, J.A.; Da, S.L.; Santos, A.S.; et al. Aerobic exercise training protects against insulin resistance, despite Low-Sodium Diet-Induced increased inflammation and visceral adiposity. Int. J. Mol. Sci. 2024, 25, 10179. https://doi.org/10.3390/ijms251810179.
- 139.
Walton, R.G.; Dungan, C.M.; Long, D.E.; Tuggle, S.C.; Kosmac, K.; Peck, B.D.; Bush, H.M.; Villasante, T.A.; Mcgwin, G.; Windham, S.T.; et al. Metformin blunts muscle hypertrophy in response to progressive resistance exercise training in older adults: A randomized, double-blind, placebo-controlled, multicenter trial: The MASTERS trial. Aging Cell 2019, 18, e13039. https://doi.org/10.1111/acel.13039.
- 140.
Long, D.E.; Peck, B.D.; Lavin, K.M.; Dungan, C.M.; Kosmac, K.; Tuggle, S.C.; Bamman, M.M.; Kern, P.A.; Peterson, C.A. Skeletal muscle properties show collagen organization and immune cell content are associated with resistance exercise response heterogeneity in older persons. J. Appl. Physiol. 2022, 132, 1432–1447. https://doi.org/10.1152/japplphysiol.00025.2022.
- 141.
Pincu, Y.; Linden, M.A.; Zou, K.; Baynard, T.; Boppart, M.D. The effects of high fat diet and moderate exercise on TGFbeta1 and collagen deposition in mouse skeletal muscle. Cytokine 2015, 73, 23–29. https://doi.org/10.1016/j.cyto.2015.01.013.
- 142.
Li, N.; Shi, H.; Guo, Q.; Gan, Y.; Zhang, Y.; Jia, J.; Zhang, L.; Zhou, Y. Aerobic exercise prevents chronic inflammation and insulin resistance in skeletal muscle of High-Fat diet mice. Nutrients 2022, 14, 3730. https://doi.org/10.3390/nu14183730.
- 143.
Samaan, M.C.; Marcinko, K.; Sikkema, S.; Fullerton, M.D.; Ziafazeli, T.; Khan, M.I.; Steinberg, G.R. Endurance interval training in obese mice reduces muscle inflammation and macrophage content independently of weight loss. Physiol. Rep. 2014, 2, e12012. https://doi.org/10.14814/phy2.12012.
- 144.
Tam, C.S.; Sparks, L.M.; Johannsen, D.L.; Covington, J.D.; Church, T.S.; Ravussin, E. Low macrophage accumulation in skeletal muscle of obese type 2 diabetics and elderly subjects. Obesity 2012, 20, 1530–1533. https://doi.org/10.1038/oby.2012.24.
- 145.
Pellegrin, M.; Bouzourene, K.; Mazzolai, L. Exercise prior to lower extremity peripheral artery disease improves endurance capacity and hindlimb blood flow by inhibiting muscle inflammation. Front. Cardiovasc. Med. 2021, 8, 706491. https://doi.org/10.3389/fcvm.2021.706491.
- 146.
de Azambuja, G.; Jorge, C.O.; Gomes, B.B.; Lourenco, H.R.; Simabuco, F.M.; Oliveira-Fusaro, M. Regular swimming exercise prevented the acute and persistent mechanical muscle hyperalgesia by modulation of macrophages phenotypes and inflammatory cytokines via PPARgamma receptors. Brain Behav. Immun. 2021, 95, 462–476. https://doi.org/10.1016/j.bbi.2021.05.002.
- 147.
Ryrso, C.K.; Thaning, P.; Siebenmann, C.; Lundby, C.; Lange, P.; Pedersen, B.K.; Hellsten, Y.; Iepsen, U.W. Effect of endurance versus resistance training on local muscle and systemic inflammation and oxidative stress in COPD. Scand. J. Med. Sci. Sports 2018, 28, 2339–2348. https://doi.org/10.1111/sms.13227.
- 148.
Luo, L.; Liu, M.; Xie, H.; Fan, Y.; Zhang, J.; Liu, L.; Li, Y.; Zhang, Q.; Wu, J.; Jiang, C.; et al. High-Intensity interval training improves physical function, prevents muscle loss, and modulates Macrophage-Mediated inflammation in skeletal muscle of cerebral ischemic mice. Mediat. Inflamm. 2021, 2021, 1849428. https://doi.org/10.1155/2021/1849428.
- 149.
Smith, T.; Barr-Gillespie, A.E.; Klyne, D.M.; Harris, M.Y.; Amin, M.; Paul, R.W.; Cruz, G.E.; Zhao, H.; Gallagher, S.; Barbe, M.F. Forced treadmill running reduces systemic inflammation yet worsens upper limb discomfort in a rat model of work-related musculoskeletal disorders. BMC Musculoskelet. Disord. 2020, 21, 57. https://doi.org/10.1186/s12891-020-3085-z.
- 150.
Abdalla, D.R.; Aleixo, A.A.; Murta, E.F.; Michelin, M.A. Innate immune response adaptation in mice subjected to administration of DMBA and physical activity. Oncol. Lett. 2014, 7, 886–890. https://doi.org/10.3892/ol.2013.1774.
- 151.
Silveira, L.S.; Batatinha, H.; Castoldi, A.; Camara, N.; Festuccia, W.T.; Souza, C.O.; Rosa, N.J.; Lira, F.S. Exercise rescues the immune response fine-tuned impaired by peroxisome proliferator-activated receptors gamma deletion in macrophages. J. Cell. Physiol. 2019, 234, 5241–5251. https://doi.org/10.1002/jcp.27333.
- 152.
Silveira, L.S.; Biondo, L.A.; de Souza, T.A.; de Lima, J.E.; Castoldi, A.; Camara, N.; Festuccia, W.T.; Rosa-Neto, J.C.; Lira, F.S. Macrophage immunophenotype but not anti-inflammatory profile is modulated by peroxisome proliferator-activated receptor gamma (PPARgamma) in exercised obese mice. Exerc. Immunol. Rev. 2020, 26, 10–22.
- 153.
Menegali, B.T.; Nesi, R.T.; Souza, P.S.; Silva, L.A.; Silveira, P.C.; Valenca, S.S.; Pinho, R.A. The effects of physical exercise on the cigarette smoke-induced pulmonary oxidative response. Pulm. Pharmacol. Ther. 2009, 22, 567–573. https://doi.org/10.1016/j.pupt.2009.08.003.
- 154.
Toledo-Arruda, A.C.; Vieira, R.P.; Guarnier, F.A.; Suehiro, C.L.; Caleman-Neto, A.; Olivo, C.R.; Arantes, P.; Almeida, F.M.; Lopes, F.; Ramos, E.; et al. Time-course effects of aerobic physical training in the prevention of cigarette smoke-induced COPD. J. Appl. Physiol. 2017, 123, 674–683. https://doi.org/10.1152/japplphysiol.00819.2016.
- 155.
Silva-Renno, A.; Baldivia, G.C.; Oliveira-Junior, M.C.; Brandao-Rangel, M.; El-Mafarjeh, E.; Dolhnikoff, M.; Mauad, T.; Britto, J.M.; Saldiva, P.; Oliveira, L.; et al. Exercise performed concomitantly with particulate matter exposure inhibits lung injury. Int. J. Sports Med. 2018, 39, 133–140. https://doi.org/10.1055/s-0043-121147.
- 156.
Olivo, C.R.; Miyaji, E.N.; Oliveira, M.L.; Almeida, F.M.; Lourenco, J.D.; Abreu, R.M.; Arantes, P.M.; Lopes, F.D.; Martins, M.A. Aerobic exercise attenuates pulmonary inflammation induced by Streptococcus pneumoniae. J. Appl. Physiol. 2014, 117, 998–1007. https://doi.org/10.1152/japplphysiol.00290.2014.
- 157.
Shi, Y.; Liu, T.; Nieman, D.C.; Cui, Y.; Li, F.; Yang, L.; Shi, H.; Chen, P. Aerobic exercise attenuates acute lung injury through NET inhibition. Front. Immunol. 2020, 11, 409. https://doi.org/10.3389/fimmu.2020.00409.
- 158.
Teixeira, R.B.; Zimmer, A.; Godoy, A.; de Castro, A.L.; Campos-Carraro, C.; Bello-Klein, A.; Da, R.A.A. Thyroid hormone treatment improved the response to maximum exercise test and preserved the ventricular geometry in myocardial infarcted rats. Exp. Physiol. 2020, 105, 1561–1570. https://doi.org/10.1113/EP088614.
- 159.
Fernandes, P.; de Mendonca, O.L.; Bruggemann, T.R.; Sato, M.N.; Olivo, C.R.; Arantes-Costa, F.M. Physical exercise induces immunoregulation of TREG, m2, and pDCs in a lung allergic inflammation model. Front. Immunol. 2019, 10, 854. https://doi.org/10.3389/fimmu.2019.00854.
- 160.
Veldhuizen, R.; Mccaig, L.A.; Pape, C.; Gill, S.E. The effects of aging and exercise on lung mechanics, surfactant and alveolar macrophages. Exp. Lung Res. 2019, 45, 113–122. https://doi.org/10.1080/01902148.2019.1605633.
- 161.
Wang, G.; Wang, L.; Wang, X.; Ye, H.; Ni, W.; Shao, W.; Dai, C.; Liu, B. Low-intensity exercise training increases systolic function of heart and MHCII (low) cardiac resident macrophages. Heliyon 2023, 9, e22915. https://doi.org/10.1016/j.heliyon.2023.e22915.
- 162.
Pellegrin, M.; Miguet-Alfonsi, C.; Bouzourene, K.; Aubert, J.F.; Deckert, V.; Berthelot, A.; Mazzolai, L.; Laurant, P. Long-term exercise stabilizes atherosclerotic plaque in ApoE knockout mice. Med. Sci. Sports Exerc. 2009, 41, 2128–2135. https://doi.org/10.1249/MSS.0b013e3181a8d530.
- 163.
Wu, H.; Zhou, R.; Kong, H.; Yang, J.; Liu, S.; Wei, X.; Li, K.; Zhang, Y. Exercise attenuates Doxorubicin-Induced myocardial injury by inhibiting TSHR and regulating macrophage polarization through miR-30d-5p/GALNT7. J. Immunol. Res. 2024, 2024, 5562293. https://doi.org/10.1155/2024/5562293.
- 164.
Botta, A.; Laher, I.; Beam, J.; Decoffe, D.; Brown, K.; Halder, S.; Devlin, A.; Gibson, D.L.; Ghosh, S. Short term exercise induces PGC-1alpha, ameliorates inflammation and increases mitochondrial membrane proteins but fails to increase respiratory enzymes in aging diabetic hearts. PLoS ONE 2013, 8, e70248. https://doi.org/10.1371/journal.pone.0070248.
- 165.
D’Haese, S.; Claes, L.; de Laat, I.; Van Campenhout, S.; Deluyker, D.; Heeren, E.; Haesen, S.; Lambrichts, I.; Wouters, K.; Schalkwijk, C.G.; Hansen, D.; et al. Moderate-Intensity and High-Intensity interval exercise training offer equal cardioprotection, with different mechanisms, during the development of type 2 diabetes in rats. Nutrients 2024, 16, 431. https://doi.org/10.3390/nu16030431.
- 166.
Singh, M.P.; Singh, G.; Singh, S.M. Role of host’s antitumor immunity in exercise-dependent regression of murine T-cell lymphoma. Comp. Immunol. Microbiol. Infect. Dis. 2005, 28, 231–248. https://doi.org/10.1016/j.cimid.2005.02.001.
- 167.
Verma, V.K.; Singh, V.; Singh, M.P.; Singh, S.M. Treadmill exercise-dependent tumor growth retardation in T-cell lymphoma-bearing host displays gender dimorphism. Oncol. Res. 2010, 18, 293–304. https://doi.org/10.3727/096504010x12629634366142.
- 168.
Almeida, P.W.; Gomes-Filho, A.; Ferreira, A.J.; Rodrigues, C.E.; Dias-Peixoto, M.F.; Russo, R.C.; Teixeira, M.M.; Cassali, G.D.; Ferreira, E.; Santos, I.C.; et al. Swim training suppresses tumor growth in mice. J. Appl. Physiol. 2009, 107, 261–265. https://doi.org/10.1152/japplphysiol.00249.2009.
- 169.
Kim, M.K.; Kim, Y.; Park, S.; Kim, E.; Kim, Y.; Kim, Y.; Kim, J.H. Effects of steady Low-Intensity exercise on High-Fat diet stimulated breast cancer progression via the alteration of macrophage polarization. Integr. Cancer Ther. 2020, 19, 1872168110. https://doi.org/10.1177/1534735420949678.
- 170.
Luo, Z.; Mei, J.; Wang, X.; Wang, R.; He, Z.; Geffen, Y.; Sun, X.; Zhang, X.; Xu, J.; Wan, R.; et al. Voluntary exercise sensitizes cancer immunotherapy via the collagen inhibition-orchestrated inflammatory tumor immune microenvironment. Cell Rep. 2024, 43, 114697. https://doi.org/10.1016/j.celrep.2024.114697.
- 171.
Savage, H.; Pareek, S.; Lee, J.; Ballaro, R.; Conterno, M.D.; Hayek, K.; Sadullozoda, M.; Lochmann, B.S.; Mcquade, J.L.; Lavoy, E.C.; et al. Aerobic exercise alters the melanoma microenvironment and modulates ERK5 s496 phosphorylation. Cancer Immunol. Res. 2023, 11, 1168–1183. https://doi.org/10.1158/2326-6066.CIR-22-0465.
- 172.
Hojman, P.; Stagaard, R.; Adachi-Fernandez, E.; Deshmukh, A.S.; Mund, A.; Olsen, C.H.; Keller, L.; Pedersen, B.K.; Gehl, J. Exercise suppresses tumor growth independent of high fat food intake and associated immune dysfunction. Sci. Rep. 2022, 12, 5476. https://doi.org/10.1038/s41598-022-08850-5.
- 173.
Rincon-Castanedo, C.; Martin-Ruiz, A.; Zazo, S.; Luis, H.A.; Valenzuela, P.L.; Moran, M.; Fleck, S.J.; Santos-Lozano, A.; Ramirez, M.; Rojo, F.; et al. Combined exercise intervention in a mouse model of high-risk neuroblastoma: Effects on physical, immune, tumor and clinical outcomes. Exerc. Immunol. Rev. 2023, 29, 86–110.
- 174.
Ge, Z.; Wu, S.; Qi, Z.; Ding, S. Exercise modulates polarization of TAMs and expression of related immune checkpoints in mice with lung cancer. J. Cancer 2022, 13, 3297–3307. https://doi.org/10.7150/jca.76136.
- 175.
Murugathasan, M.; Jafari, A.; Amandeep, A.; Hassan, S.A.; Chihata, M.; Abdul-Sater, A.A. Moderate exercise induces trained immunity in macrophages. Am. J. Physiol. Cell Physiol. 2023, 325, C429–C442. https://doi.org/10.1152/ajpcell.00130.2023.
- 176.
Moraes, M.R.; Rosa, T.S.; Souza, M.K.; Neves, R.; Bacurau, R.; Passos, C.S.; Cenedeze, M.A.; Passos, M.T.; Machado, F.G.; Pacheco-Silva, F.A.; et al. Resistance training downregulates macrophages infiltration in the kidney of 5/6 nephrectomized rats. Life Sci. 2018, 213, 190–197. https://doi.org/10.1016/j.lfs.2018.10.037.
- 177.
Valkenborghs, S.R.; Wood, L.G.; Callister, R.; Upham, J.W.; Grainge, C.L.; Anderson, S.; Williams, L.M.; Mcloughlin, R.F.; Williams, E.J.; Scott, H.A. Effects of moderate- versus Vigorous-Intensity exercise training on asthma outcomes in adults. J. Allergy Clin. Immunol. Pract. 2024, 12, 2744–2753. https://doi.org/10.1016/j.jaip.2024.06.015.
- 178.
Marinho, R.; Munoz, V.R.; Pauli, L.; Ropelle, E.; de Moura, L.P.; Moraes, J.C.; Moura-Assis, A.; Cintra, D.E.; Da, S.A.; Ropelle, E.R.; et al. Endurance training prevents inflammation and apoptosis in hypothalamic neurons of obese mice. J. Cell. Physiol. 2018, 234, 880–890. https://doi.org/10.1002/jcp.26909.
- 179.
Hespe, G.E.; Kataru, R.P.; Savetsky, I.L.; Garcia, N.G.; Torrisi, J.S.; Nitti, M.D.; Gardenier, J.C.; Zhou, J.; Yu, J.Z.; Jones, L.W.; et al. Exercise training improves obesity-related lymphatic dysfunction. J. Physiol. 2016, 594, 4267–4282. https://doi.org/10.1113/JP271757.
- 180.
Trott, D.W.; Henson, G.D.; Ho, M.; Allison, S.A.; Lesniewski, L.A.; Donato, A.J. Age-related arterial immune cell infiltration in mice is attenuated by caloric restriction or voluntary exercise. Exp. Gerontol. 2018, 109, 99–107. https://doi.org/10.1016/j.exger.2016.12.016.
- 181.
Chrysostomou, V.; Kezic, J.M.; Trounce, I.A.; Crowston, J.G. Forced exercise protects the aged optic nerve against intraocular pressure injury. Neurobiol. Aging 2014, 35, 1722–1725. https://doi.org/10.1016/j.neurobiolaging.2014.01.019.
- 182.
Uchida, M.; Horii, N.; Hasegawa, N.; Fujie, S.; Oyanagi, E.; Yano, H.; Iemitsu, M. Gene expression profiles for macrophage in tissues in response to different exercise training protocols in senescence mice. Front. Sports Act Living 2019, 1, 50. https://doi.org/10.3389/fspor.2019.00050.
- 183.
Zaychik, Y.; Fainstein, N.; Touloumi, O.; Goldberg, Y.; Hamdi, L.; Segal, S.; Nabat, H.; Zoidou, S.; Grigoriadis, N.; Katz, A.; et al. High-Intensity exercise training protects the brain against autoimmune neuroinflammation: Regulation of microglial redox and pro-inflammatory functions. Front. Cell. Neurosci. 2021, 15, 640724. https://doi.org/10.3389/fncel.2021.640724.
- 184.
Soto, I.; Graham, L.C.; Richter, H.J.; Simeone, S.N.; Radell, J.E.; Grabowska, W.; Funkhouser, W.K.; Howell, M.C.; Howell, G.R. APOE stabilization by exercise prevents aging neurovascular dysfunction and complement induction. PLoS Biol. 2015, 13, e1002279. https://doi.org/10.1371/journal.pbio.1002279.
- 185.
Wang, W.; Lv, Z.; Gao, J.; Liu, M.; Wang, Y.; Tang, C.; Xiang, J. Treadmill exercise alleviates neuronal damage by suppressing NLRP3 inflammasome and microglial activation in the MPTP mouse model of Parkinson’s disease. Brain Res. Bull. 2021, 174, 349–358. https://doi.org/10.1016/j.brainresbull.2021.06.024.
- 186.
Chhaya, S.J.; Quiros-Molina, D.; Tamashiro-Orrego, A.D.; Houle, J.D.; Detloff, M.R. Exercise-Induced changes to the macrophage response in the dorsal root ganglia prevent neuropathic pain after spinal cord injury. J. Neurotrauma 2019, 36, 877–890. https://doi.org/10.1089/neu.2018.5819.
- 187.
Grace, P.M.; Fabisiak, T.J.; Green-Fulgham, S.M.; Anderson, N.D.; Strand, K.A.; Kwilasz, A.J.; Galer, E.L.; Walker, F.R.; Greenwood, B.N.; Maier, S.F.; et al. Prior voluntary wheel running attenuates neuropathic pain. Pain 2016, 157, 2012–2023. https://doi.org/10.1097/j.pain.0000000000000607.
- 188.
Bobinski, F.; Teixeira, J.M.; Sluka, K.A.; Santos, A. Interleukin-4 mediates the analgesia produced by low-intensity exercise in mice with neuropathic pain. Pain 2018, 159, 437–450. https://doi.org/10.1097/j.pain.0000000000001109.
- 189.
Klein, D.; Yuan, X.; Weiss, E.M.; Martini, R. Physical exercise mitigates neuropathic changes in an animal model for Charcot-Marie-Tooth disease 1X. Exp. Neurol. 2021, 343, 113786. https://doi.org/10.1016/j.expneurol.2021.113786.
- 190.
Davaa, G.; Hong, J.Y.; Kim, T.U.; Lee, S.J.; Kim, S.Y.; Hong, K.; Hyun, J.K. Exercise ameliorates spinal cord injury by changing DNA methylation. Cells 2021, 10, 143. https://doi.org/10.3390/cells10010143.
- 191.
Jablonski, K.; Young, N.A.; Henry, C.; Caution, K.; Kalyanasundaram, A.; Okafor, I.; Harb, P.; Schwarz, E.; Consiglio, P.; Cirimotich, C.M.; et al. Physical activity prevents acute inflammation in a gout model by downregulation of TLR2 on circulating neutrophils as well as inhibition of serum CXCL1 and is associated with decreased pain and inflammation in gout patients. PLoS ONE 2020, 15, e237520. https://doi.org/10.1371/journal.pone.0237520.
- 192.
Shen, P.; Jia, S.; Wang, Y.; Zhou, X.; Zhang, D.; Jin, Z.; Wang, Z.; Liu, D.; Bai, L.; Yang, Y. Mechanical stress protects against chondrocyte pyroptosis through lipoxin A(4) via synovial macrophage M2 subtype polarization in an osteoarthritis model. Biomed. Pharmacother. 2022, 153, 113361. https://doi.org/10.1016/j.biopha.2022.113361.
- 193.
Oka, Y.; Murata, K.; Ozone, K.; Minegishi, Y.; Kano, T.; Shimada, N.; Kanemura, N. Mild treadmill exercise inhibits cartilage degeneration via macrophages in an osteoarthritis mouse model. Osteoarthr. Cartil. Open 2023, 5, 100359. https://doi.org/10.1016/j.ocarto.2023.100359.
- 194.
Chen, L.; Zhang, M.; Yang, X.; Wang, Y.; Huang, T.; Li, X.; Ban, Y.; Li, Q.; Yang, Q.; Zhang, Y.; et al. Methyl-CpG-binding 2 K271 lactylation-mediated M2 macrophage polarization inhibits atherosclerosis Theranostics. 2024, 14, 4256–4277. https://doi.org/10.7150/thno.94738.
- 195.
Bresler, A.; Vogel, J.; Niederer, D.; Gray, D.; Schmitz-Rixen, T.; Troidl, K. Development of an exercise training protocol to investigate arteriogenesis in a murine model of peripheral artery disease. Int. J. Mol. Sci. 2019, 20, 3956. https://doi.org/10.3390/ijms20163956.
- 196.
Baltgalvis, K.A.; Berger, F.G.; Pena, M.M.; Davis, J.M.; Carson, J.A. Effect of exercise on biological pathways in ApcMin/+ mouse intestinal polyps. J. Appl. Physiol. 2008, 104, 1137–1143. https://doi.org/10.1152/japplphysiol.00955.2007.
- 197.
Mcclellan, J.L.; Steiner, J.L.; Day, S.D.; Enos, R.T.; Davis, M.J.; Singh, U.P.; Murphy, E.A. Exercise effects on polyp burden and immune markers in the ApcMin/+ mouse model of intestinal tumorigenesis. Int. J. Oncol. 2014, 45, 861–868. https://doi.org/10.3892/ijo.2014.2457.
- 198.
Kawanishi, M.; Kami, K.; Nishimura, Y.; Minami, K.; Senba, E.; Umemoto, Y.; Kinoshita, T.; Tajima, F. Exercise-induced increase in M2 macrophages accelerates wound healing in young mice. Physiol. Rep. 2022, 10, e15447. https://doi.org/10.14814/phy2.15447.
- 199.
Keylock, K.T.; Vieira, V.J.; Wallig, M.A.; Dipietro, L.A.; Schrementi, M.; Woods, J.A. Exercise accelerates cutaneous wound healing and decreases wound inflammation in aged mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R179–R184. https://doi.org/10.1152/ajpregu.00177.2007.
- 200.
Leandro, C.G.; de Lima, T.M.; Alba-Loureiro, T.C.; Do, N.E.; Manhaes, D.C.R.; de Castro, C.M.; Pithon-Curi, T.C.; Curi, R. Stress-induced downregulation of macrophage phagocytic function is attenuated by exercise training in rats. Neuroimmunomodulation 2007, 14, 4–7. https://doi.org/10.1159/000107282.
- 201.
Dos, S.R.; Caperuto, E.C.; de Mello, M.T.; Costa, R.L. Effect of exercise on glutamine metabolism in macrophages of trained rats. Eur. J. Appl. Physiol. 2009, 107, 309–315. https://doi.org/10.1007/s00421-009-1130-6.
- 202.
Komine, S.; Akiyama, K.; Warabi, E.; Oh, S.; Kuga, K.; Ishige, K.; Togashi, S.; Yanagawa, T.; Shoda, J. Exercise training enhances in vivo clearance of endotoxin and attenuates inflammatory responses by potentiating Kupffer cell phagocytosis. Sci. Rep. 2017, 7, 11977. https://doi.org/10.1038/s41598-017-12358-8.
- 203.
Meneguello-Coutinho, M.; Caperuto, E.; Bacurau, A.V.; Chamusca, G.; Uchida, M.C.; Tibana, R.A.; Pereira, G.B.; Navalta, J.W.; Wasinski, F.; Cavaglieri, C.R.; et al. Effects of dietary restriction or swimming on lymphocytes and macrophages functionality from old rats. Immunol. Investig. 2014, 43, 113–122. https://doi.org/10.3109/08820139.2013.847456.
- 204.
Zhao, F.; Pang, W.; Zhang, Z.; Zhao, J.; Wang, X.; Liu, Y.; Wang, X.; Feng, Z.; Zhang, Y.; Sun, W.; et al. Pomegranate extract and exercise provide additive benefits on improvement of immune function by inhibiting inflammation and oxidative stress in high-fat-diet-induced obesity in rats. J. Nutr. Biochem. 2016, 32, 20–28. https://doi.org/10.1016/j.jnutbio.2016.02.003.
- 205.
Miura, I.; Komine, S.; Okada, K.; Wada, S.; Warabi, E.; Uchida, F.; Oh, S.; Suzuki, H.; Mizokami, Y.; Shoda, J. Prevention of non-alcoholic steatohepatitis by long-term exercise via the induction of phenotypic changes in Kupffer cells of hyperphagic obese mice. Physiol. Rep. 2021, 9, e14859. https://doi.org/10.14814/phy2.14859.
- 206.
Oh, S.; So, R.; Shida, T.; Matsuo, T.; Kim, B.; Akiyama, K.; Isobe, T.; Okamoto, Y.; Tanaka, K.; Shoda, J. High-Intensity aerobic exercise improves both hepatic fat content and stiffness in sedentary obese men with nonalcoholic fatty liver disease. Sci. Rep. 2017, 7, 43029. https://doi.org/10.1038/srep43029.
- 207.
Estruel-Amades, S.; Camps-Bossacoma, M.; Massot-Cladera, M.; Perez-Cano, F.J.; Castell, M. Alterations in the innate immune system due to exhausting exercise in intensively trained rats. Sci. Rep. 2020, 10, 967. https://doi.org/10.1038/s41598-020-57783-4.
- 208.
Xiao, W.; Chen, P.; Liu, X.; Zhao, L. The impaired function of macrophages induced by strenuous exercise could not be ameliorated by BCAA supplementation. Nutrients 2015, 7, 8645–8656. https://doi.org/10.3390/nu7105425.
- 209.
Xiao, W.; Chen, P.; Dong, J.; Wang, R.; Luo, B. Dietary glutamine supplementation partly reverses impaired macrophage function resulting from overload training in rats. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 179–187. https://doi.org/10.1123/ijsnem.2014-0118.
- 210.
Xiao, W.; Chen, P.; Wang, R.; Dong, J. Overload training inhibits phagocytosis and ROS generation of peritoneal macrophages: Role of IGF-1 and MGF. Eur. J. Appl. Physiol. 2013, 113, 117–125. https://doi.org/10.1007/s00421-012-2418-5.
- 211.
Shi, Y.; Cai, D.; Wang, X.; Liu, X. Immunomodulatory effect of ganoderma lucidum polysaccharides (GLP) on long-term heavy-load exercising mice. Int. J. Vitam. Nutr. Res. 2012, 82, 383–390. https://doi.org/10.1024/0300-9831/a000135.
- 212.
van de Weert-Van, L.P.; de Vrankrijker, A.M.; Fentz, J.; Ciofu, O.; Wojtaszewski, J.F.; Arets, H.G.; Hulzebos, H.J.; van der Ent, C.K.; Beekman, J.M.; Johansen, H.K. Effect of long-term voluntary exercise wheel running on susceptibility to bacterial pulmonary infections in a mouse model. PLoS ONE 2013, 8, e82869. https://doi.org/10.1371/journal.pone.0082869.
- 213.
Galvez, I.; Martin-Cordero, L.; Hinchado, M.D.; Alvarez-Barrientos, A.; Ortega, E. Obesity Affects beta2 Adrenergic Regulation of the Inflammatory Profile and Phenotype of Circulating Monocytes from Exercised Animals. Nutrients 2019, 11, 2630. https://doi.org/10.3390/nu11112630.
- 214.
Shirato, K.; Imaizumi, K.; Sakurai, T.; Ogasawara, J.; Ohno, H.; Kizaki, T. Regular voluntary exercise potentiates interleukin-1beta and interleukin-18 secretion by increasing caspase-1 expression in murine macrophages. Mediat. Inflamm. 2017, 2017, 9290416. https://doi.org/10.1155/2017/9290416.
- 215.
Kizaki, T.; Takemasa, T.; Sakurai, T.; Izawa, T.; Hanawa, T.; Kamiya, S.; Haga, S.; Imaizumi, K.; Ohno, H. Adaptation of macrophages to exercise training improves innate immunity. Biochem. Biophys. Res. Commun. 2008, 372, 152–156. https://doi.org/10.1016/j.bbrc.2008.05.005.
- 216.
Terra, R.; Alves, P.J.; Goncalves, D.S.S.; Salerno, V.P.; Dutra, P.M. Exercise improves the Th1 response by modulating cytokine and NO production in BALB/c mice. Int. J. Sports Med. 2013, 34, 661–666. https://doi.org/10.1055/s-0032-1329992.
- 217.
Garcia, J.J.; Martin-Cordero, L.; Hinchado, M.D.; Bote, M.E.; Ortega, E. Effects of habitual exercise on the eHsp72-induced release of inflammatory cytokines by macrophages from obese Zucker rats. Int. J. Sports Med. 2013, 34, 559–564. https://doi.org/10.1055/s-0032-1327650.
- 218.
Van Pelt, D.W.; Guth, L.M.; Horowitz, J.F. Aerobic exercise elevates markers of angiogenesis and macrophage IL-6 gene expression in the subcutaneous adipose tissue of overweight-to-obese adults. J. Appl. Physiol. 2017, 123, 1150–1159. https://doi.org/10.1152/japplphysiol.00614.2017.
- 219.
Kizaki, T.; Maegawa, T.; Sakurai, T.; Ogasawara, J.E.; Ookawara, T.; Oh-Ishi, S.; Izawa, T.; Haga, S.; Ohno, H. Voluntary exercise attenuates obesity-associated inflammation through ghrelin expressed in macrophages. Biochem. Biophys. Res. Commun. 2011, 413, 454–459. https://doi.org/10.1016/j.bbrc.2011.08.117.
- 220.
Batista, M.J.; Santos, R.V.; Oliveira, E.M.; Seelaender, M.C.; Costa, R.L. Endurance training restores peritoneal macrophage function in post-MI congestive heart failure rats. J. Appl. Physiol. 2007, 102, 2033–2039. https://doi.org/10.1152/japplphysiol.00871.2006.
- 221.
Javaid, H.; Sahar, N.E.; Zhuge, D.L.; Huh, J.Y. Exercise inhibits NLRP3 inflammasome activation in obese mice via the Anti-Inflammatory effect of meteorin-like. Cells 2021, 10, 3480. https://doi.org/10.3390/cells10123480.
- 222.
Frellstedt, L.; Waldschmidt, I.; Gosset, P.; Desmet, C.; Pirottin, D.; Bureau, F.; Farnir, F.; Franck, T.; Dupuis-Tricaud, M.C.; Lekeux, P.; et al. Training modifies innate immune responses in blood monocytes and in pulmonary alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 2014, 51, 135–142. https://doi.org/10.1165/rcmb.2013-0341OC.
- 223.
Ortega, E.; Bote, M.E.; Giraldo, E.; Garcia, J.J. Aquatic exercise improves the monocyte pro- and anti-inflammatory cytokine production balance in fibromyalgia patients. Scand. J. Med. Sci. Sports 2012, 22, 104–112. https://doi.org/10.1111/j.1600-0838.2010.01132.x.
- 224.
Khosravi, N.; Hanson, E.D.; Farajivafa, V.; Evans, W.S.; Lee, J.T.; Danson, E.; Wagoner, C.W.; Harrell, E.P.; Sullivan, S.A.; Nyrop, K.A.; et al. Exercise-induced modulation of monocytes in breast cancer survivors. Brain Behav. Immun. Health 2021, 14, 100216. https://doi.org/10.1016/j.bbih.2021.100216.
- 225.
Belotto, M.F.; Magdalon, J.; Rodrigues, H.G.; Vinolo, M.A.; Curi, R.; Pithon-Curi, T.C.; Hatanaka, E. Moderate exercise improves leucocyte function and decreases inflammation in diabetes. Clin. Exp. Immunol. 2010, 162, 237–243. https://doi.org/10.1111/j.1365-2249.2010.04240.x.
- 226.
Mauer, J.; Denson, J.L.; Bruning, J.C. Versatile functions for IL-6 in metabolism and cancer. Trends Immunol. 2015, 36, 92–101. https://doi.org/10.1016/j.it.2014.12.008.
- 227.
Nguyen, K.D.; Qiu, Y.; Cui, X.; Goh, Y.P.; Mwangi, J.; David, T.; Mukundan, L.; Brombacher, F.; Locksley, R.M.; Chawla, A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 2011, 480, 104–108. https://doi.org/10.1038/nature10653.
- 228.
Qiu, Y.; Nguyen, K.D.; Odegaard, J.I.; Cui, X.; Tian, X.; Locksley, R.M.; Palmiter, R.D.; Chawla, A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 2014, 157, 1292–1308. https://doi.org/10.1016/j.cell.2014.03.066.
- 229.
Koivisto, V.A.; Soman, V.R.; Felig, P. Effects of acute exercise on insulin binding to monocytes in obesity. Metabolism 1980, 29, 168–172. https://doi.org/10.1016/0026-0495(80)90142-0.
- 230.
Soman, V.R.; Koivisto, V.A.; Deibert, D.; Felig, P.; Defronzo, R.A. Increased insulin sensitivity and insulin binding to monocytes after physical training. N. Engl. J. Med. 1979, 301, 1200–1204. https://doi.org/10.1056/NEJM197911293012203.
- 231.
Pedersen, O.; Beck-Nielsen, H.; Heding, L. Increased insulin receptors after exercise in patients with insulin-dependent diabetes mellitus. N. Engl. J. Med. 1980, 302, 886–892. https://doi.org/10.1056/NEJM198004173021603.
- 232.
Koivisto, V.A.; Soman, V.; Conrad, P.; Hendler, R.; Nadel, E.; Felig, P. Insulin binding to monocytes in trained athletes: Changes in the resting state and after exercise. J. Clin. Investig. 1979, 64, 1011–1015. https://doi.org/10.1172/JCI109537.
- 233.
Robinson, A.; Han, C.Z.; Glass, C.K.; Pollard, J.W. Monocyte regulation in homeostasis and malignancy. Trends Immunol. 2021, 42, 104–119. https://doi.org/10.1016/j.it.2020.12.001.
- 234.
Cassetta, L.; Pollard, J.W. A timeline of tumour-associated macrophage biology. Nat. Rev. Cancer 2023, 23, 238–257. https://doi.org/10.1038/s41568-022-00547-1.
- 235.
Mantovani, A.; Marchesi, F.; Jaillon, S.; Garlanda, C.; Allavena, P. Tumor-associated myeloid cells: Diversity and therapeutic targeting. Cell. Mol. Immunol. 2021, 18, 566–578. https://doi.org/10.1038/s41423-020-00613-4.
- 236.
Toledo, B.; Zhu, C.L.; Paniagua-Sancho, M.; Marchal, J.A.; Peran, M.; Giovannetti, E. Deciphering the performance of macrophages in tumour microenvironment: A call for precision immunotherapy. J. Hematol. Oncol. 2024, 17, 44.
- 237.
Koelwyn, G.J.; Wennerberg, E.; Demaria, S.; Jones, L.W. Exercise in regulation of Inflammation-Immune axis function in cancer initiation and progression. Oncology 2015, 29, 214800.
- 238.
Liu, Y.; Xu, R.; Gu, H.; Zhang, E.; Qu, J.; Cao, W.; Huang, X.; Yan, H.; He, J.; Cai, Z. Metabolic reprogramming in macrophage responses. Biomark. Res. 2021, 9, 1. https://doi.org/10.1186/s40364-020-00251-y.
- 239.
Calderin, E.P.; Zheng, J.J.; Boyd, N.L.; Mcnally, L.; Audam, T.N.; Lorkiewicz, P.; Hill, B.G.; Hellmann, J. Exercise-induced specialized proresolving mediators stimulate AMPK phosphorylation to promote mitochondrial respiration in macrophages. Mol. Metab. 2022, 66, 101637. https://doi.org/10.1016/j.molmet.2022.101637.
- 240.
Silvin, A.; Qian, J.; Ginhoux, F.; Brain macrophage development, diversity and dysregulation in health and disease. Cell. Mol. Immunol. 2023, 20, 1277–1289. https://doi.org/10.1038/s41423-023-01053-6.
- 241.
Cheon, J.; Kwon, S.; Kim, M. Exerkines mitigating Alzheimer’s disease progression by regulating inflammation: Focusing on macrophage/microglial NLRP3 inflammasome pathway. Alzheimers Dement. 2025, 21, e14432. https://doi.org/10.1002/alz.14432.
- 242.
Damier, P.; Hirsch, E.C.; Agid, Y.; Graybiel, A.M. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 1999, 122, 1437–1448. https://doi.org/10.1093/brain/122.8.1437.
- 243.
Ouchi, Y.; Yoshikawa, E.; Sekine, Y.; Futatsubashi, M.; Kanno, T.; Ogusu, T.; Torizuka, T. Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann. Neurol. 2005, 57, 168–175. https://doi.org/10.1002/ana.20338.
- 244.
Chen, Y.; Sun, Y.; Luo, Z.; Chen, X.; Wang, Y.; Qi, B.; Lin, J.; Lin, W.W.; Sun, C.; Zhou, Y.; et al. Exercise modifies the transcriptional regulatory features of monocytes in alzheimer’s patients: A Multi-Omics integration analysis based on single cell technology. Front. Aging Neurosci. 2022, 14, 881488. https://doi.org/10.3389/fnagi.2022.881488.
- 245.
Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; van Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007, 204, 1057–1069. https://doi.org/10.1084/jem.20070075.
- 246.
Segawa, M.; Fukada, S.; Yamamoto, Y.; Yahagi, H.; Kanematsu, M.; Sato, M.; Ito, T.; Uezumi, A.; Hayashi, S.; Miyagoe-Suzuki, Y.; et al. Suppression of macrophage functions impairs skeletal muscle regeneration with severe fibrosis. Exp. Cell Res. 2008, 314, 3232–3244. https://doi.org/10.1016/j.yexcr.2008.08.008.
- 247.
Stromberg, A.; Olsson, K.; Dijksterhuis, J.P.; Rullman, E.; Schulte, G.; Gustafsson, T. CX3CL1—A macrophage chemoattractant induced by a single bout of exercise in human skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R297–R304. https://doi.org/10.1152/ajpregu.00236.2015.
- 248.
Yu, S.H.; Huang, C.Y.; Lee, S.D.; Hsu, M.F.; Wang, R.Y.; Kao, C.L.; Kuo, C.H. Decreased eccentric exercise-induced macrophage infiltration in skeletal muscle after supplementation with a class of ginseng-derived steroids. PLoS ONE 2014, 9, e114649. https://doi.org/10.1371/journal.pone.0114649.
- 249.
Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. https://doi.org/10.1038/s41574-022-00641-2.
- 250.
Rao, R.R.; Long, J.Z.; White, J.P.; Svensson, K.J.; Lou, J.; Lokurkar, I.; Jedrychowski, M.P.; Ruas, J.L.; Wrann, C.D.; Lo, J.C.; et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 2014, 157, 1279–1291. https://doi.org/10.1016/j.cell.2014.03.065.
- 251.
Zeng, H.; Liu, X.; Liu, P.; Jia, S.; Wei, G.; Chen, G.; Zhao, L. Exercise’s protective role in chronic obstructive pulmonary disease via modulation of M1 macrophage phenotype through the miR-124-3p/ERN1 axis. Sci. Prog. 2025, 108, 352331388. https://doi.org/10.1177/00368504251360892.
- 252.
Pedersen, L.; Idorn, M.; Olofsson, G.H.; Lauenborg, B.; Nookaew, I.; Hansen, R.H.; Johannesen, H.H.; Becker, J.C.; Pedersen, K.S.; Dethlefsen, C.; et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-Dependent NK cell mobilization and redistribution. Cell Metab. 2016, 23, 554–562. https://doi.org/10.1016/j.cmet.2016.01.011.
- 253.
Zhang, N.; Wang, X.; Feng, M.; Li, M.; Wang, J.; Yang, H.; He, S.; Xia, Z.; Shang, L.; Jiang, X.; et al. Early-life exercise induces immunometabolic epigenetic modification enhancing anti-inflammatory immunity in middle-aged male mice. Nat. Commun. 2024, 15, 3103.
- 254.
Li, V.L.; He, Y.; Contrepois, K.; Liu, H.; Kim, J.T.; Wiggenhorn, A.L.; Tanzo, J.T.; Tung, A.S.; Lyu, X.; Zushin, P.H.; et al. An exercise-inducible metabolite that suppresses feeding and obesity. Nature 2022, 606, 785–790. https://doi.org/10.1038/s41586-022-04828-5.
- 255.
Yu, R.; Zhang, C.; Yuan, M.; Ye, S.; Hu, T.; Chen, S.; Liang, G.; Liu, J.; Ke, H.; Huang, J.; et al. Exercise-induced metabolite n-lactoyl-phenylalanine ameliorates colitis by inhibiting m1 macrophage polarization via the suppression of the NF-kappaB signaling pathway. Cell Mol. Gastroenterol. Hepatol. 2025, 19, 101558. https://doi.org/10.1016/j.jcmgh.2025.101558.
- 256.
Colegio, O.R.; Chu, N.Q.; Szabo, A.L.; Chu, T.; Rhebergen, A.M.; Jairam, V.; Cyrus, N.; Brokowski, C.E.; Eisenbarth, S.C.; Phillips, G.M.; et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 2014, 513, 559–563. https://doi.org/10.1038/nature13490.
- 257.
Mills, E.L.; Ryan, D.G.; Prag, H.A.; Dikovskaya, D.; Menon, D.; Zaslona, Z.; Jedrychowski, M.P.; Costa, A.; Higgins, M.; Hams, E.; et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 2018, 556, 113–117. https://doi.org/10.1038/nature25986.
- 258.
Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Dabritz, J.; Gottlieb, E.; Latorre, I.; et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 2016, 167, 457–470. https://doi.org/10.1016/j.cell.2016.08.064.
- 259.
Liu, P.S.; Wang, H.; Li, X.; Chao, T.; Teav, T.; Christen, S.; Di Conza, G.; Cheng, W.C.; Chou, C.H.; Vavakova, M.; et al. Alpha-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat. Immunol. 2017, 18, 985–994. https://doi.org/10.1038/ni.3796.
- 260.
Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-Mcdermott, E.M.; Mcgettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature 2013, 496, 238–242. https://doi.org/10.1038/nature11986.
- 261.
Cereijo, R.; Gavalda-Navarro, A.; Cairo, M.; Quesada-Lopez, T.; Villarroya, J.; Moron-Ros, S.; Sanchez-Infantes, D.; Peyrou, M.; Iglesias, R.; Mampel, T.; et al. CXCL14, a brown adipokine that mediates Brown-Fat-to-Macrophage communication in thermogenic adaptation. Cell Metab. 2018, 28, 750–763. https://doi.org/10.1016/j.cmet.2018.07.015.
- 262.
Hui, X.; Gu, P.; Zhang, J.; Nie, T.; Pan, Y.; Wu, D.; Feng, T.; Zhong, C.; Wang, Y.; Lam, K.S.; et al. Adiponectin enhances Cold-Induced browning of subcutaneous adipose tissue via promoting m2 macrophage proliferation. Cell Metab. 2015, 22, 279–290. https://doi.org/10.1016/j.cmet.2015.06.004.
- 263.
Huang, Z.; Zhong, L.; Lee, J.; Zhang, J.; Wu, D.; Geng, L.; Wang, Y.; Wong, C.M.; Xu, A. The FGF21-CCL11 axis mediates beiging of white adipose tissues by coupling sympathetic nervous system to type 2 immunity. Cell Metab. 2017, 26, 493–508. https://doi.org/10.1016/j.cmet.2017.08.003.
- 264.
Tu, Y.; Liu, J.; Kong, D.; Guo, X.; Li, J.; Long, Z.; Peng, J.; Wang, Z.; Wu, H.; Liu, P.; et al. Irisin drives macrophage anti-inflammatory differentiation via JAK2-STAT6-dependent activation of PPARgamma and Nrf2 signaling. Free Radic. Biol. Med. 2023, 201, 98–110. https://doi.org/10.1016/j.freeradbiomed.2023.03.014.
- 265.
Bosma, M.; Gerling, M.; Pasto, J.; Georgiadi, A.; Graham, E.; Shilkova, O.; Iwata, Y.; Almer, S.; Soderman, J.; Toftgard, R.; et al. FNDC4 acts as an anti-inflammatory factor on macrophages and improves colitis in mice. Nat. Commun. 2016, 7, 11314. https://doi.org/10.1038/ncomms11314.
- 266.
Graff, R.M.; Kunz, H.E.; Agha, N.H.; Baker, F.L.; Laughlin, M.; Bigley, A.B.; Markofski, M.M.; Lavoy, E.C.; Katsanis, E.; Bond, R.A.; et al. β2-Adrenergic receptor signaling mediates the preferential mobilization of differentiated subsets of CD8+ T-cells, NK-cells and non-classical monocytes in response to acute exercise in humans. Brain Behav. Immun. 2018, 74, 143–153. https://doi.org/10.1016/j.bbi.2018.08.017.
- 267.
Liu, Z.; Jian, C.; Yuan, W.; Jia, G.; Cheng, D.; Liu, Y.; Zhang, Y.; Zhang, B.; Zhou, Z.; Zhao, G. Epinephrine promotes tumor progression and M2 polarization of tumor-associated macrophages by regulating the TRIM2- NF-kappaB pathway in colorectal cancer cells. Genes Dis. 2024, 11, 101092. https://doi.org/10.1016/j.gendis.2023.101092.
- 268.
Okutsu, M.; Suzuki, K.; Ishijima, T.; Peake, J.; Higuchi, M. The effects of acute exercise-induced cortisol on CCR2 expression on human monocytes. Brain Behav. Immun. 2008, 22, 1066–1071. https://doi.org/10.1016/j.bbi.2008.03.006.
- 269.
Toobian, D.; Ghosh, P.; Katkar, G.D. Parsing the role of PPARs in macrophage processes. Front. Immunol. 2021, 12, 783780. https://doi.org/10.3389/fimmu.2021.783780.
- 270.
Odegaard, J.I.; Ricardo-Gonzalez, R.R.; Goforth, M.H.; Morel, C.R.; Subramanian, V.; Mukundan, L.; Red, E.A.; Vats, D.; Brombacher, F.; Ferrante, A.W.; et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 2007, 447, 1116–1120. https://doi.org/10.1038/nature05894.
- 271.
Bouhlel, M.A.; Derudas, B.; Rigamonti, E.; Dievart, R.; Brozek, J.; Haulon, S.; Zawadzki, C.; Jude, B.; Torpier, G.; Marx, N.; et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007, 6, 137–143. https://doi.org/10.1016/j.cmet.2007.06.010.
- 272.
Cheng, W.Y.; Huynh, H.; Chen, P.; Pena-Llopis, S.; Wan, Y. Macrophage PPARgamma inhibits Gpr132 to mediate the anti-tumor effects of rosiglitazone. eLife 2016, 5, e18501. https://doi.org/10.7554/eLife.18501.
- 273.
Yang, X.; Deng, B.; Zhao, W.; Guo, Y.; Wan, Y.; Wu, Z.; Su, S.; Gu, J.; Hu, X.; Feng, W.; et al. FABP5+ lipid-loaded macrophages process tumour-derived unsaturated fatty acid signal to suppress T-cell antitumour immunity. J. Hepatol. 2025, 82, 676–689. https://doi.org/10.1016/j.jhep.2024.09.029.
- 274.
Sippel, T.R.; Johnson, A.M.; Li, H.Y.; Hanson, D.; Nguyen, T.T.; Bullock, B.L.; Poczobutt, J.M.; Kwak, J.W.; Kleczko, E.K.; Weiser-Evans, M.C.; et al. Activation of PPARgamma in myeloid cells promotes progression of epithelial lung tumors through TGFbeta1. Mol. Cancer Res. 2019, 17, 1748–1758. https://doi.org/10.1158/1541-7786.MCR-19-0236.
- 275.
Butcher, L.R.; Thomas, A.; Backx, K.; Roberts, A.; Webb, R.; Morris, K. Low-intensity exercise exerts beneficial effects on plasma lipids via PPARgamma. Med. Sci. Sports Exerc. 2008, 40, 1263–1270. https://doi.org/10.1249/MSS.0b013e31816c091d.
- 276.
Thomas, A.W.; Davies, N.A.; Moir, H.; Watkeys, L.; Ruffino, J.S.; Isa, S.A.; Butcher, L.R.; Hughes, M.G.; Morris, K.; Webb, R. Exercise-associated generation of PPARgamma ligands activates PPARgamma signaling events and upregulates genes related to lipid metabolism. J. Appl. Physiol. 2012, 112, 806–815. https://doi.org/10.1152/japplphysiol.00864.2011.
- 277.
Yakeu, G.; Butcher, L.; Isa, S.; Webb, R.; Roberts, A.W.; Thomas, A.W.; Backx, K.; James, P.E.; Morris, K. Low-intensity exercise enhances expression of markers of alternative activation in circulating leukocytes: Roles of PPARgamma and Th2 cytokines. Atherosclerosis 2010, 212, 668–673. https://doi.org/10.1016/j.atherosclerosis.2010.07.002.
- 278.
Mcgee, S.L.; Hargreaves, M. Exercise adaptations: Molecular mechanisms and potential targets for therapeutic benefit. Nat. Rev. Endocrinol. 2020, 16, 495–505. https://doi.org/10.1038/s41574-020-0377-1.

This work is licensed under a Creative Commons Attribution 4.0 International License.


