Downloads
Download


This work is licensed under a Creative Commons Attribution 4.0 International License.
Article
A Minimally Invasive Approach for Cardiac Electrophysiology Studies in Mice
Min Zi , * , Sabu Abraham , Alicia D'souza , David Hutchings , Sukhpal Prehar , Xin Wang , and Elizabeth J Cartwright
Division of Cardiovascular Sciences, Faculty of Biology, Medicine and Health, University of Manchester, Manchester M13 9PL, United Kingdom
* Correspondence: min.zi@manchester.ac.uk
Received: 6 January 2023
Accepted: 9 February 2023
Published: 25 March 2023
Abstract: Atrial fibrillation and ventricular tachycardia are commonly seen in clinic. Different approaches have been developed to investigate underlying mechanisms. Transvenous approach (TA) is widely used for studies but has several drawbacks. We therefore developed a novel minimally invasive approach (MIA) for mechanistic studies. Study included 27 male C57BL/6J mice, 19 for MIA and 8 for TA. Under general anaesthesia, ECG was recorded. A key hole was made on the right first intercostal space by separating the intercostal muscles, followed by the exposure of the superior vena cava and the top of the atrium. An EPR-800 catheter was inserted vertically, perpendicular to the chest, for atrial pacing and flatly over the ventricles for ventricular pacing. Burst S1–S1 and decremental S1–S2 pacing protocols were performed to evaluate SA recovery time (SNRT), the atrioventricular node effective refractory period (AVN-ERP), Wenckebach period, ventricular ERP, and arrhythmia susceptibility. MIA was successfully performed in all 19 mice without any complications. One mouse died during TA due to venous rupture. Compared MIA with TA, surgical time were significantly shorter (P<0.0001). Wenckebach period was shorter as well (P<0.05). No difference was found in baseline sinus cycle length, SNRT, correct SNRT, AVN-ERP, ventricular ERP, and arrhythmia susceptibility (all P>0.05). The novel MIA outplays TA by providing similar outcomes of PES but consuming less time, demanding less surgical expertise, and reducing the potential of surgical complications. Given the minimal tissue injury, it also provides great potential as a recovery procedure for longitudinal study.
Keywords:
cardiovascular disease arrhythmia electrophysiology programmed electrical stimulation mouse minimally invasive1. Introduction
Heartbeat is controlled by the cardiac conduction system, which consists of the sinus node (SAN) in the right atrium, the atrioventricular node (AVN) on the right side of the atrial septum near the junction of the atria and the ventricles, the bundle of His and the purkinje fibres in the ventricles. The primary electrical activities originate from pacemaker cells in the SAN and then spread down to the AVN, the bundle of His and the purkinje fibres consecutively to initiate myocardial contraction. A stable and rhythmic heartbeat is fundamental in order to maintain normal blood circulation for life. Any disruption to these electrical activities will result in arrhythmia, which is usually classified by the site of origin. Of these, atrial fibrillation (AF) is the most common in clinical practice. It affects approximately 2.7 to 6.1 million people in the USA and around 1.4 million people in England each year [ 1]. The prevalence increases with age, affecting about 10% of the population aged 80 years and over [ 1‒ 4]. Both in symptomatic and asymptomatic cases, AF is an independent risk factor for stroke, myocardial infarction, heart failure, and sudden death [ 5‒ 8]. Ventricular arrhythmias, including premature ventricular contraction, ventricular tachycardia (VT) and ventricular fibrillation (VF), are commonly seen in patients with cardiovascular diseases and associated with high mortality due to cardiac sudden death and heart failure [ 9‒ 12].
Cardiac arrhythmia is associated with a spectrum of clinical conditions, such as ischemic and congenital heart diseases, hypertension, metabolic and immunological diseases, infections, excessive alcohol consumption and certain drugs, etc. The underlying mechanism is largely unknown. With the development of genetically engineered mouse models in the past few decades, some techniques for programmed electrical stimulation (PES) on mouse atria and ventricles have been developed to investigate molecular mechanisms underlying AF and ventricular arrhythmia. In 1996, a technique of epicardial PES via open chest was reported by Berul et al [13], which allows direct pacing at different points on the cardiac surface. Thereafter, an endocardial pacing technique via a transvenous route was reported [14,15], followed by routes for transesophageal pacing [ 16] and subdiaphragmatic ventricular stimulation [ 17]. Each route has different pros and cons. Studies using open chest epicardial pacing and transvenous endocardial pacing can provide more information than other approaches because the pacing catheter can contact both atrium and ventricles. In particular, the transvenous approach enables in vivo studies to assess the function of the cardiac conduction system from SAN, AVN to the bundle of His and Purkinje fibres in the ventricles [ 15, 18]. However, these methods aren’t suitable for longitudinal studies because animals cannot be recovered due to substantial injuries to the chest and major blood vessels sustained during these procedures. Transesophageal pacing is a non-invasive procedure, which allows animals to survive. It, therefore, can be used for repeated assessment of therapeutic effectiveness over a longer period. The drawbacks of this technique however include an inability to pace the ventricles and lack of direct contact with the right atrium. The subsequent distance between pacing electrodes and the atrium may therefore induce artefacts obscuring the atrial complex, affecting the reliability of the measurement of atrial effective refractory period (ERP) [ 19].
Given the circumstances, a recovery pacing procedure that allows direct contact to the right atrium and ventricles would be desirable, particularly for drug development that usually requires a longitudinal assessment of therapy. We therefore developed a minimally invasive epicardial technique for mechanistic investigation of both atrial and ventricular conduction dysfunction and arrhythmia susceptibility. Due to the nature of minimal invasiveness, this approach has a great potential of animal recovery for repeated assessment when necessary. By comparing with the commonly used PES via a transvenous route, its advantages in surgical technique and reliability in electrophysiological assessment have been confirmed.
2. Methods and Materials
2.1. Animals
C57BL/6J male mice (Charles River Laboratories, UK) were used in the present study. All mice were 8‒10 weeks old, weighing 23–31 g, acclimating for 1 week before the experiment. The animals were housed in a temperature and humidity-controlled room, with free access to food and water, and 12 hours light/dark cycles. All surgical procedures were performed under the project license (PP5982529) in accordance with the United Kingdom Scientific Procedures Act 1986, approved by the Ethics Committee of the University of Manchester. All surgical procedures and PES were performed by an experienced researcher.
2.2. Determination of A Minimally Invasive Route for Epicardial Atrial and Ventricular Pacing
Following a Schedule 1 procedure, the chest was widely opened at the midline to visualize the position of heart (n=3). As shown in Figure 1A, the right atrium occupied the second intercostal space on the right site of the sternum. The ventricles lay inferior on the left side of sternum. To confirm this in live animals, echocardiography was performed using a Vevo 3100 Preclinical Imaging System (FUJIFILM VisuaSonic) under 1.5% isoflurane (n=4). As shown in Figure 1A and 1B, an ultra-high frequency transducer (MX700) was placed at the position of the first and second intercostal spaces, at a 15 degree angle to the axillary line in order to get a parasternal short axis view of the right atrium. The right atrium was observed. Therefore, to place an electrode catheter into the first intercostal space vertically, perpendicular to the chest, on the right side of the sternum will allow it to attach to the connection area of the superior vena cava and right atrium (Figure 1C and 1D), where the SAN is located. To insert the catheter flatly, parallel to the chest, through the first right intercostal space and down the left side of the sternum, it will place the catheter on the surface of heart, allowing direct contact to the ventricles for ventricular pacing ( Figure 1E). Because the catheter is moved in the mediastinum, it won’t affect the intactness of the pleural cavity. Therefore, ventilation is not required.
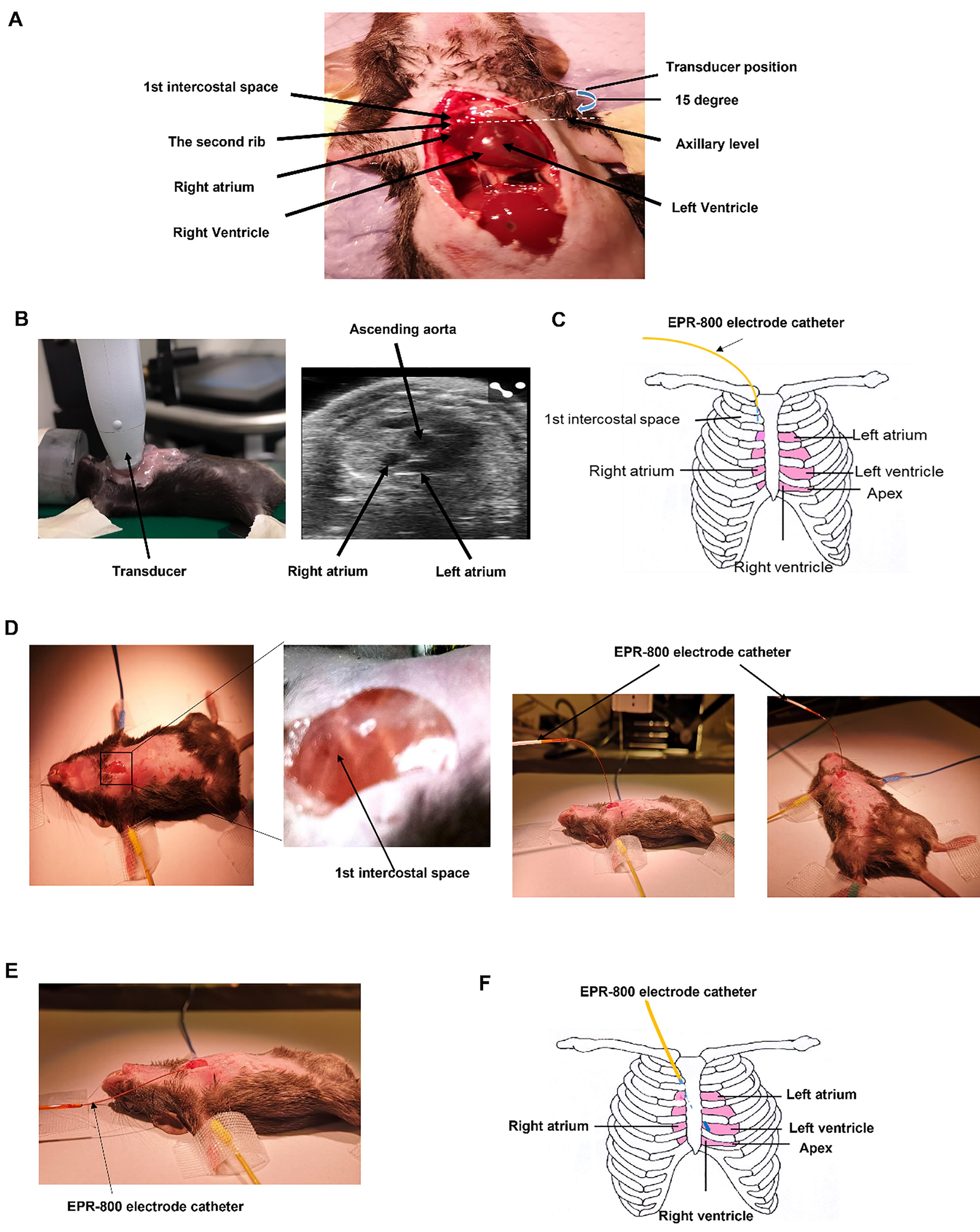
Figure 1. Determination of pacing route. A. Anatomical study of the position of the right atrium and ventricles. B. Position of the right atrium in the parasternal short axis view of echocardiography. C. Illustration of the route of minimally invasive epicardia pacing. The pacing catheter was placed through the first right intercostal space. D. Step by step surgical procedure. The EPR-800 electrode catheter was placed vertically into the first intercostal space for atrial pacing, 6 of 8 electrodes inside the chest. E. For ventricular pacing, the EPR-800 catheter was placed flatly through the first right intercostal space, slightly towards the left side of the sternum. All 8 electrodes were inserted into the chest. F. Illustration of the catheter position.
2.3. Surgical Procedure
According to our data obtained from studies conducted over the past 10 years, isoflurane can induce cardiac arrhythmia in 5‒10% of mice undergoing echocardiography and electrocardiogram (ECG) examinations (data not shown). This side effect may obscure the arrhythmia susceptibility in programmed electrophysiology study. Therefore, we recommend other anaesthetics for PES in mice such as ketamine/xylazine (100‒200 mg/kg body weight for Ketamine and 5‒16 mg/kg for xylazine) for recovery procedures, or avertin (250 mg/kg) for non-recovery procedure. The present study was designed as a terminal procedure under our animal licence. Therefore, avertin was used for anaesthesia.
After anaesthesia, a surface 3-lead ECG was obtained by placing 27 gauge needles subcutaneously on 3 limbs (both forelimbs and the right hind leg). ECG trace was recorded during PES using a PowerLab/4SP (ADInstruments) and analyzed using LabChart 8 software (ADInstruments). An 8-mm incision was made at the axillary level on the right side of the sternum. Then, a key hole was made at the first intercostal space by separating the intercostal muscles using two Dumont #7b forceps (InterFocus Ltd, Cambridge), followed by the exposure of the superior vena cava and the top of atrium. An EPR-800 electrode catheter (Millar instrument) was inserted vertically for atrial pacing ( Figure 1D) and flatly over the ventricles in the mediastinum for ventricular pacing ( Figure 1E).
2.4. Programmed Electrophysiology Study
2.4.1. Protocols
Two types of pacing protocols were used to evaluate conduction function and arrhythmia susceptibility. The first was a burst S1‒S1 protocol, which involves the delivery of a series of impulses (S1) at a constant rate. The second was a decremental S1‒S2 protocol, which consisted of an initial drive train of 8 stimuli (S1) at a fixed rate, followed by an extra stimulus (S2). While the drive train repeated, the S1‒S2 coupling interval was reduced by 2ms each time until ventricular conduction was lost.
2.4.2. Equipment Settings
Programmed electrophysiology study was conducted using a DS3 isolated constant-current stimulator (Digitimer Ltd.) that connected with a CED Micro3-1401 data acquisition system, controlled by Spike software (Cambridge Electronic Design). The pulse duration was set as 2ms. Using a 50-beat S1‒S1 protocol with spontaneous sinus cycle length as S1‒S1 interval, the threshold was gradually increased from 1mA until it captured the atrium. This threshold was determined as the minimum threshold. Then, the stimulation threshold was set as 0.5 mA higher than the minimum threshold, which enabled continuous capture of cardiac conduction with a cycle length of 100 ms. This threshold was kept constant for all subsequent pacing protocols.
2.4.3. Electrophysiology Study Details
The study aimed to evaluate cardiac conduction function and arrhythmia susceptibility. Cardiac conduction properties included SA recovery time (SNRT) and the correct SNRT (cSNRT) for SAN function, AVN-ERP and Wenckebach period for AVN refractoriness and conduction, and ventricular ERP. SNRT and Wenckebach period were measured using a 50-beat S1-S1 burst pacing with various cycle lengths (from 100 ms to 60 ms, 2 ms decrement each time). SNRT was defined as the interval between the last captured atrial pacing stimulus and the first recovered sinus P wave. The cSNRT was calculated as SNRT minus the baseline sinus cycle length before rapid atrial pacing. The longest SNRT and cSNRT were used for final analysis. Wenckebach conduction appeared when the pacing cycle length was reduced to certain level (usually below 85 ms). The P:QRS ratio was gradually reduced with the reduction of stimulation cycle length. Finally, when P:QRS ratio became 2:1, the stimulation cycle length was counted as Wenckebach cycle length for final analysis. AVN and ventricular ERPs were determined using S1‒S2 decremental pacing. The interval for S1 stimuli was fixed at 100ms. The S1‒S2 interval was gradually reduced from 100 ms. The shortest S1‒S2 interval that led to the last atrial or ventricular conduction by S2 was defined as AVN-ERP or ventricular ERP. Representative ECG traces for these measurements are shown in Figure 2.
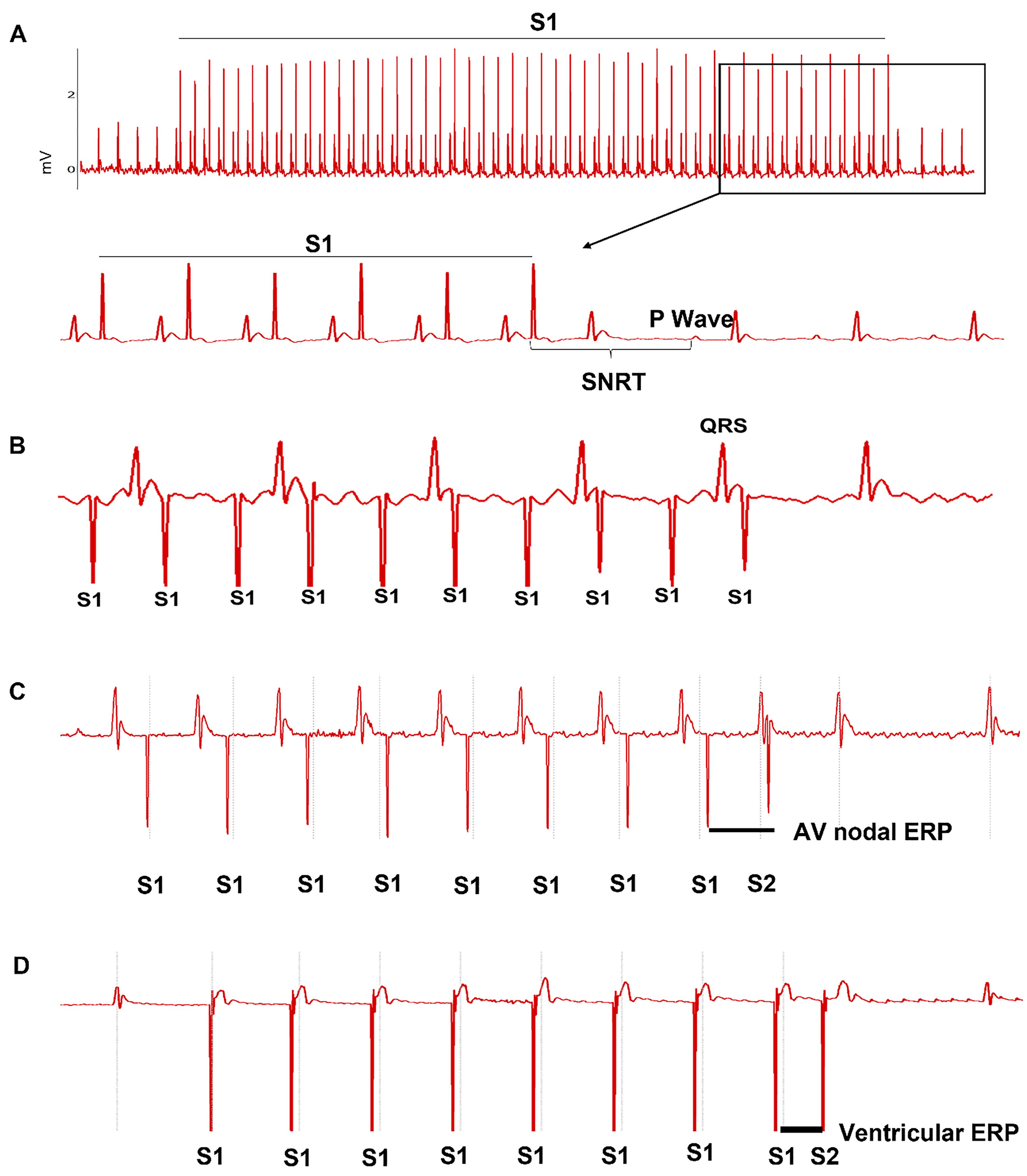
Figure 2. Atrial and ventricular epicardial pacing using a minimally invasive approach. A. Sinus node recovery time (SNRT) was determined using S1-S1 pacing protocol. B. Wenckebach 1∶1 atrial to ventricular conduction. C. Atrial effective refractory period (ERP) was determined using S1‒S2 pacing protocol. D. Ventricular effective refractory period (ERP) was determined using S1‒S2 pacing protocol.
Arrhythmia susceptibility was assessed using the above S1‒S2 decremental pacing protocol and a fast S1‒S1 burst pacing (cycle length: 50 ms, 40 ms and 30 ms, respectively). Each S1‒S1 pacing train lasted for 20 s. The recovery interval was at least 30 s between pacing trains [ 20]. AF was defined if more than 0.5 s of continuous high frequency and polymorphic P waves appeared after termination of pacing. VT was defined when more than three wide QRS complexes presented consecutively after termination of pacing. Representative ECG traces are shown in Figure 3.

Figure 3. Arrhythmia susceptibility evaluated using minimally invasive epicardial pacing. A. Multiple episodes of atrial fibrillation (AF) induced by S1‒S2 protocol in one mouse. B. AF induced by S1‒S1 protocol with 50 ms cycle length. C. Polymorphic ventricular tachycardia (VT) was induced by S1‒S2 protocol. D. Polymorphic VT was induced by S1‒S1 protocol with 50 ms cycle length.
2.5. Electrophysiology Study Via the Transvenous Endocardial Approach
Another group of mice underwent atrial and ventricular pacing via a transvenous approach as described previously [14,15]. Under a surgical microscope, the right jugular vein was isolated and a small transversal opening (±0.5 mm) was made on the vein, followed by inserting an EPR-800 electrode catheter through the right jugular vein into the right atrium and ventricle ( Figure 4A). The PES protocols for the transvenous approach were identical to those for the minimally invasive approach. Representative ECG traces are shown in Figure 4B‒4E for cardiac conduction properties and Figure 5 for atrial and ventricular tachycardia induction. Data obtained from this approach were used to verify the minimally invasive approach.

Figure 4. Atrial and ventricular endocardial pacing via an intravenous approach. A. Illustration of the catheter position. B. Sinus node recovery time (SNRT) was determined using S1‒S1 pacing protocol. C. Wenckebach phenomenon mixed with 1∶1, 2∶1, and 4∶1 ratio of atrial to ventricular conduction. D. Atrial effective refractory period (ERP) was determined using S1‒S2 pacing protocol. E. Ventricular effective refractory period (ERP) was determined using S1‒S2 pacing protocol.
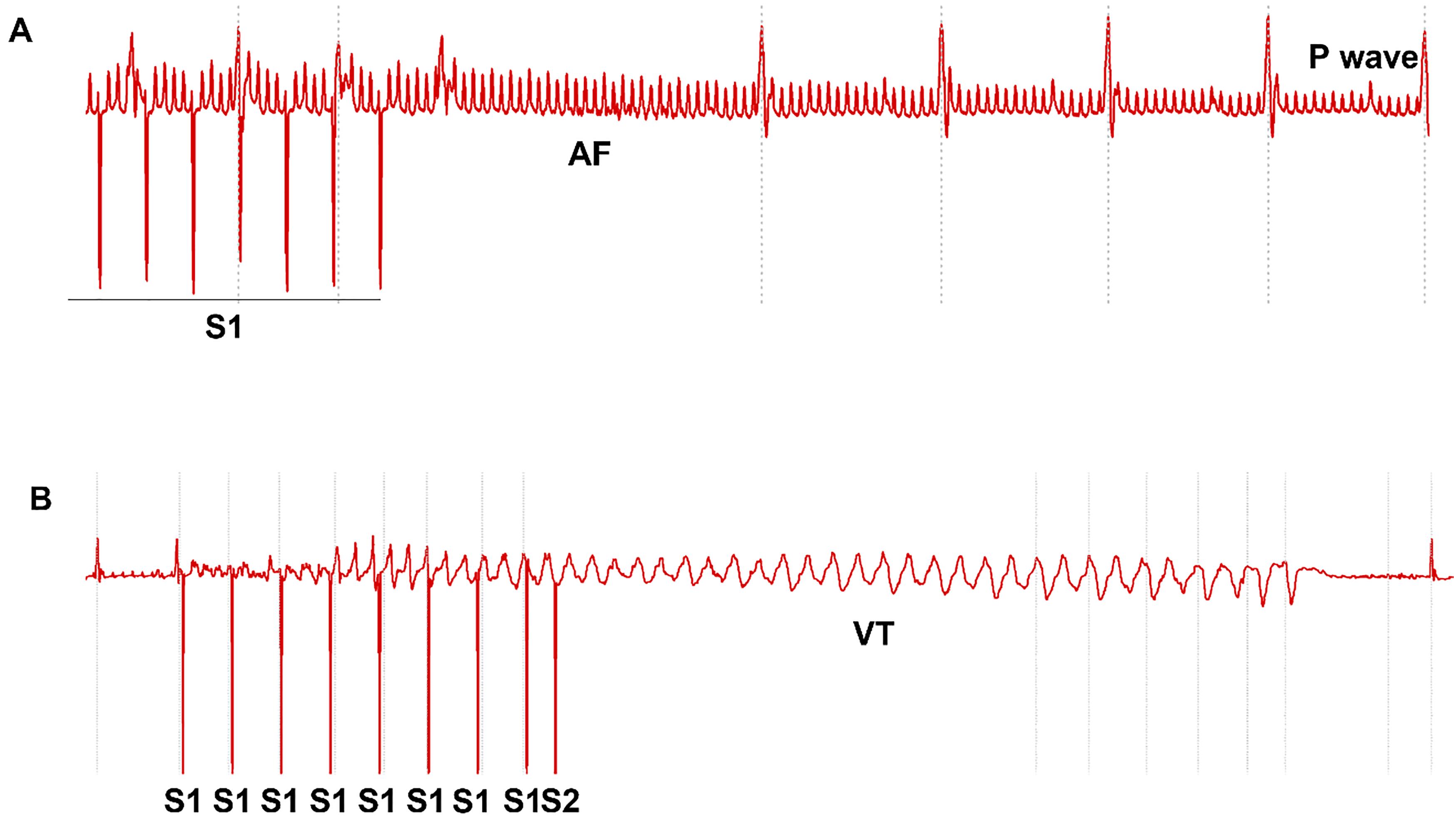
Figure 5. Arrhythmia susceptibility evaluated using intravenous endocardial pacing. A. S1‒S1 atrial pacing induced a short period of atrial fibrillation (AF). B. Polymorphic ventricular tachycardia (VT) induced by S1‒S2 protocol.
2.6. Statistical Analysis
Data are presented as mean ± S.E.M. or n (%), and analyzed using IBM SPSS 25.0 software. Two-tail Student’s t-test and Chi square test are used for the comparisons between the two approaches. P value<0.05 is considered statistically significant.
3. Results
3.1. The Technical Evaluation of Minimally Invasive Epicardial Pacing
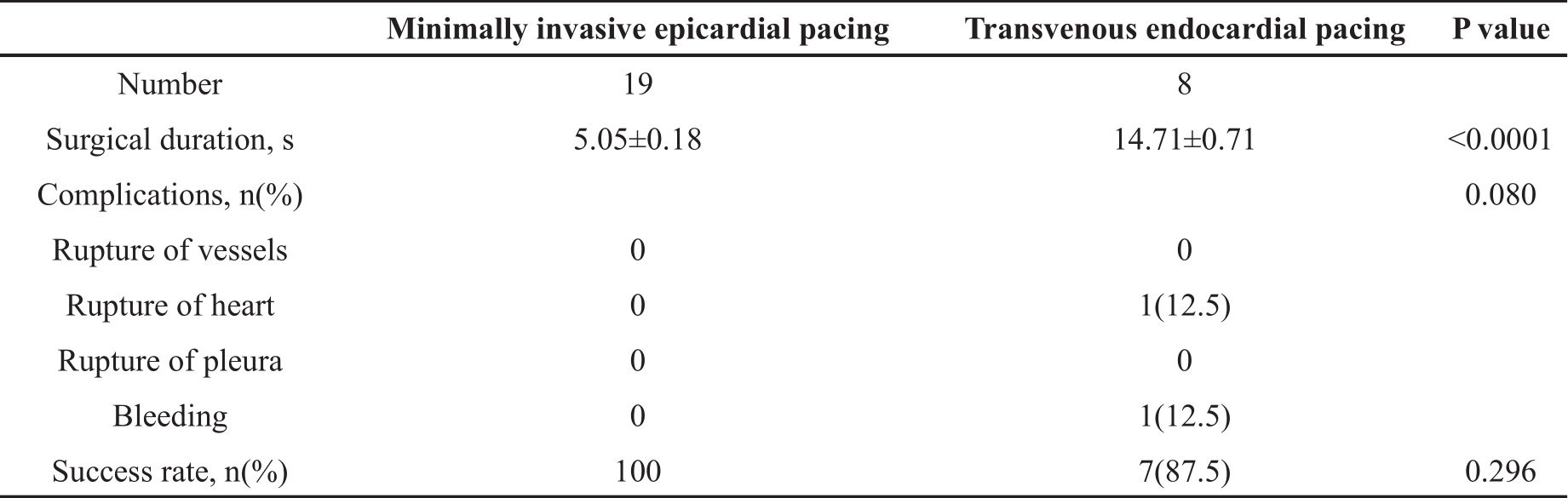
A total of 19 mice underwent the minimally invasive epicardial pacing. The procedure for placing the catheter in the correct location was straightforward without repeated attempts. The animals were not ventilated because the pleural cavity was kept intact via this route. All mice successfully completed the study without any complications such as bleeding, cardiovascular or pleural ruptures, or death. Another 8 mice underwent the transvenous endocardial pacing. Repeated attempts were required to correct the catheter position in one mouse. Complications included atrial perforation and bleeding from jugular vein. There was one death due to the atrial perforation. Comparing the two groups, the mean surgical duration was significantly shorter for the minimally invasive approach (P<0.0001). Although the success rate was not different (P=0.296), there was a trend of more complications with the transvenous approach (P=0.080). All these data indicated that the surgical procedure for the minimally invasive approach is less time-consuming and relatively safe ( Table 1).
Table 1. Technical comparison between the transvenous and minimally invasive approaches.
3.2. Functional Evaluation of Cardiac Conduction System
Once the pacing catheter was in the correct position, atrial and ventricular conductions were captured and evaluated sequentially. As shown in Table 2, comparing the minimally invasive approach with the transvenous approach, baseline sinus cycle length, SNRT, CSNRT and Ventricular ERP were not significantly different (all P>0.05). However, Wenckebach cycle length obtained by the minimally invasive approach was slightly but significantly shorter than those obtained by the transvenous approach (P<0.05). Similarly, AVN ERP induced by the minimally invasive approach tended to be shorter (P=0.06 compared the two approaches). These indicated that the minimally invasive approach was less sensitive to induce 2:1 AV block than the transvenous approach.
Table 2. Comparison of electrophysiology data obtained via transvenous and minimally invasive approaches.
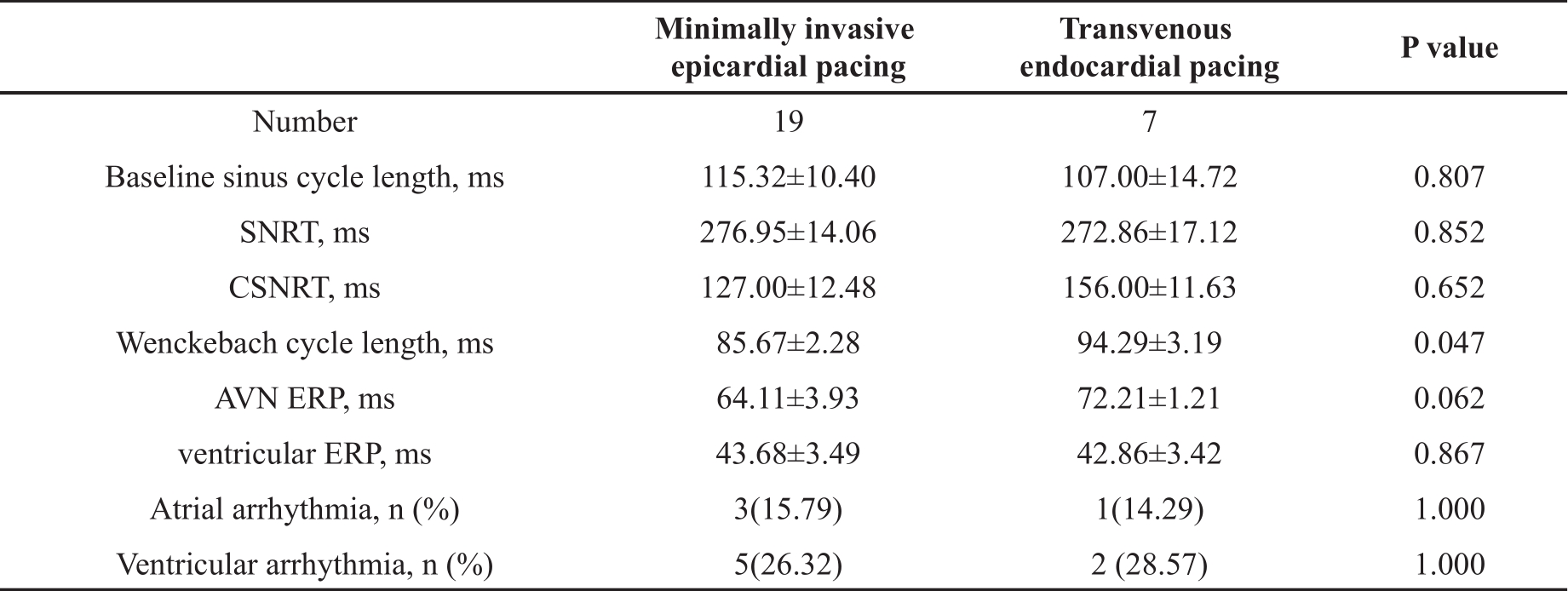
SNRT, sinus node recovery time; CSNRT, corrected sinus node recovery time; AVN, atrioventricular node; ERP, effective refractory period; ms, millisecond. Data presented as mean ± SEM or n (%).
3.3. Arrhythmia Susceptibility Assessment
The assessment results about arrhythmia susceptibility were included in Table 2. Using different burst S1‒S1 pacing protocols (cycle length: 50ms, 40ms and 30ms, respectively) and a decremental S1‒S2 pacing protocol, the provocation of atrial and ventricular arrhythmia was attempted. Of 19 mice undergoing the minimally invasive pacing, AF was induced in 3 mice (15.79%). One mouse had multiple episodes of AF and the longest episode lasted for 2.5 s. The other two mice had a single episode of AF, lasting for 1 second each. In the mice undergoing the transvenous pacing, one of seven mice (14.29%) had a single episode of AF, lasting for less than 1 s. No difference was found in the frequency of AF induction between the two approaches (P>0.05). In all cases, AF was induced by 20-s burst S1‒S1 pacing with 50 ms cycle length.
Following fast ventricular pacing, 5 of 19 mice (26.32%) undergoing the minimally invasive approach had multiple episodes of VT, induced by S1‒S2 protocol only in 3 mice and by both S1‒S2 and S1‒S1 protocols in 2 mice. VT was polymorphic in 3 mice stimulated by S1‒S2 pacing and the longest episode lasted for 2 s. In mice paced via the transvenous route, a single episode of polymorphic VT was induced by S1‒S2 protocol in 2 mice (28.57%). The longest one lasted for 0.5 s. On the whole, no difference was found in the frequency of VT induction between the two approaches (P>0.05) ( Table 1). S1‒S2 protocol tends to be more sensitive to trigger ventricular arrhythmia than S1-S1 protocol (7/26 vs. 2/26, P=0.06).
4. Discussion
In the present study, we developed a minimally invasive epicardial pacing approach for functional assessment of the cardiac conduction system and arrhythmia susceptibility. This minimally invasive approach produced similar outcomes of PES to the commonly used transvenous approach, except shorter Wenckebach cycle length for 2∶1 AV block. It provided considerable advantages over the transvenous approach by consuming less time, demanding less surgical expertise, and reducing the potential of surgical complications such as bleeding, cardiovascular and pleural ruptures, or death. Moreover, the minimal invasiveness of this approach only caused minor soft tissue injury to the chest wall, providing a great potential for animal recovery and repeated temporal measurements.
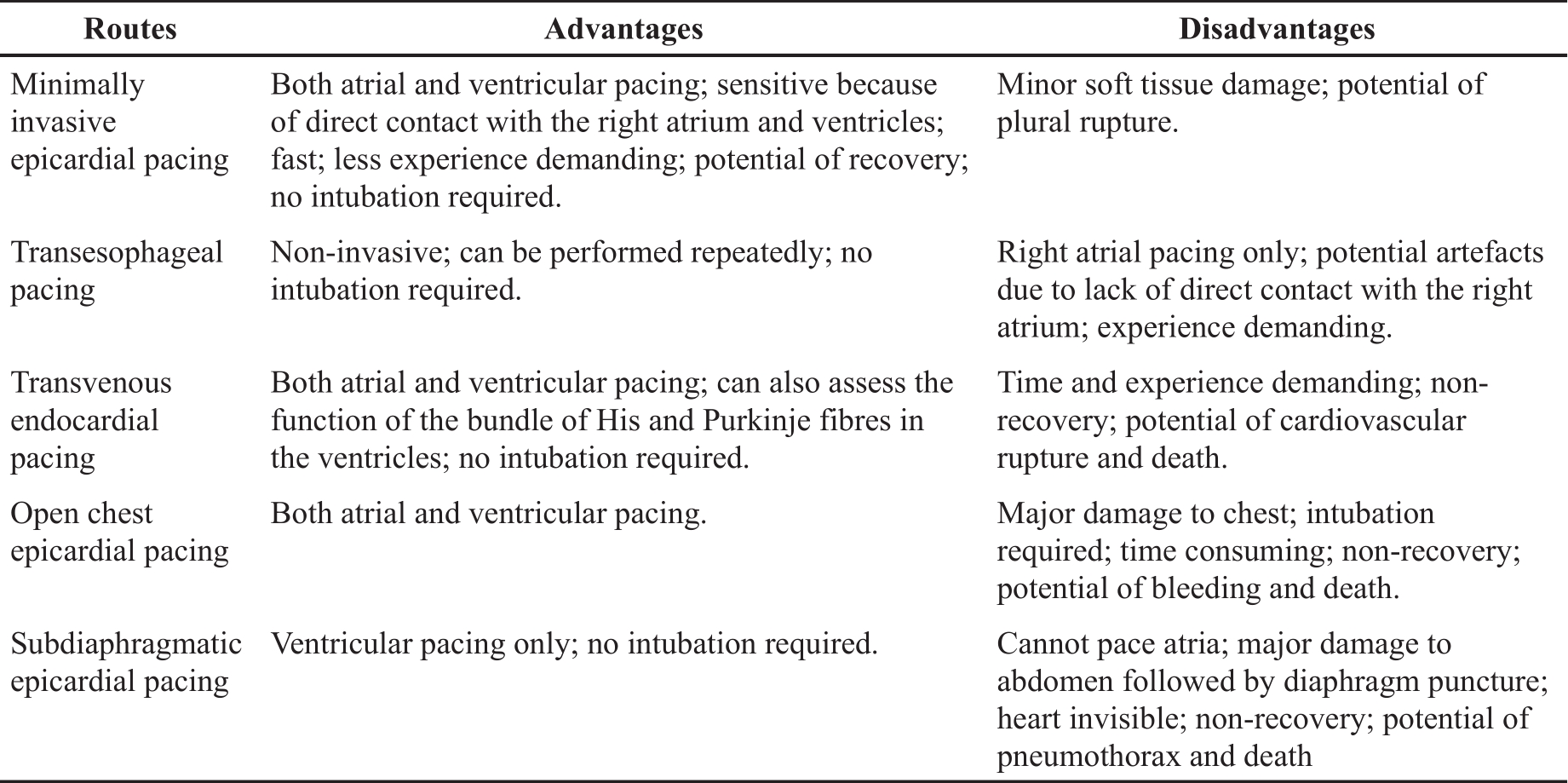
At present, several techniques for PES in mouse models are available, including epicardial pacing via open chest, subdiaphragmatic or transesophageal routes, and transvenous endocardial pacing via the jugular vein. The advantages and disadvantages of each route has been listed in Table 3. Of them, the transvenous endocardial pacing via the jugular vein has been widely used because it enables functional studies to assess the whole cardiac conduction system from SAN, AVN to the bundle of His and Purkinje fibres in ventricles [ 18]. The drawbacks of this technique include the need for practical experience, the time-consuming while advancing and positioning the catheter to properly capture atrium and ventricle, and potential complications such as bleeding, the rupture of veins or the heart, and death [ 13]. Particularly, this procedure results in permanent ligation of the jugular vein [ 21, 22]. Although occluding one of the jugular veins may have no significant impact on health [ 23], animal termination is strongly recommended given the invasive nature and considerable tissue damage surrounding the jugular vein. This approach, therefore, cannot be employed for longitudinal studies. In addition, if heart size is too small or too big, it is difficult to get the catheter into a right position inside heart, which limits the application of this technique in young mice and those with dilated hearts due to heart failure or other pathologies. However, what the minimally invasive approach achieved is over and above what is expected from the transvenous approach. On top of comparable performance in PES, the new approach can overcome all aforementioned drawbacks of the transvenous approach. Particularly, the size of heart does not matter during this new approach because the catheter does not need to enter the heart. It is very useful for PES in mice with heart failure, through minimising the stress associated with difficulties in positioning the catheter and surgical complications. Compared to transesophageal pacing, the only available non-invasive pacing for arium, our new method can directly pace both atrium and ventricle but requires less experience demanding. Although we did not recover animals at the end of the procedure due to the limitations of our current animal licence, it is unquestionable that the new method offers a great potential as a recovery procedure for longitudinal studies given the minimal effect of soft tissue injury on health.
Table 3. Comparison of different routes for atrial and ventricular pacing.
We found that Wenckebach cycle length obtained by our minimally invasive approach was slightly but significantly shorter than those obtained via the transvenous approach. The literature regarding the indication of Wenckebach cycle length in preclinical studies is very limited. In a clinical study, increased Wenckebach cycle length was associated with a high risk for AV block [ 24]. In our case, shorter Wenckebach cycle length may indicate less sensitivity for AV block provocation by the minimally invasive approach. This is likely influenced by the distance remaining between pacing electrodes and the AV node via this approach, while using the transvenous approach, the pacing electrodes are placed more closely to the AV node endocardially [ 25]. Nevertheless, AV block is still inducible using our new approach.
Studies have demonstrated the importance of heart size in arrhythmia induction [ 18]. Murine hearts have low propensity for atrial or ventricular arrhythmias inducibility because the critically small size of the heart exacerbates the difficulty to facilitate sustained re-entries for arrhythmia triggering [ 18, 26]. But our experiments and others’ have shown that atrial and ventricular arrhythmia can be induced [ 14, 16, 18, 21, 22, 25, 27]. In the present study, data derived from PES via our minimally invasive approach have demonstrated that AF is inducible in 16% of C57BL/6J mice using a fast S1‒S1 pacing protocol at stimulation cycle length of 50ms. In contrast, Schrickel et al have shown that a 15s rapid pacing via the transesophageal route can frequently induce AF in C57BL/6 mice at stimulation cycle lengths between 15‒25 ms but not at stimulation cycle lengths > 50 ms [ 16]. This may further confirm the influence of distance between pacing electrodes and the atrium on AF inducibility encountered via the transesophageal approach, which reduces the impact of stimulation on the atrium. Similarly, different pacing protocols have been created and tested for VT assessment. We found that VT could be triggered by both fast S1‒S1 (cycle length<100ms) and decremental S1‒S2 protocols, but the latter seems more sensitive, inducing VT in 26% of C57BL/6J mice. Unlike us, other researchers found that burst pacing was more aggressive in VT induction [ 28], which may be related to different mouse strains used in the studies. Many researchers tend to use extra stimuli to increase the sensitivity of pacing protocols to VT triggering [ 28‒ 30]. On the one hand, the more extra stimuli, the higher sensitivity of VT inducibility. On the other hand, the higher the sensibility, the lower the specificity of arrhythmia provocation during mechanistic study. We believe that any aggressive protocol for arrhythmia induction determined in wild type control mice will reduce the ability to detect arrhythmogenesis in genetically modified or stress-induced mice. The power of the triggering should be controlled within a reasonable scope after taking account of mouse strain and pacing approach routes. To date, we have tested this new approach and PES protocols in four additional studies with reproducible results (data not shown).
There are two limitations to the present study. First, we did not assess the new minimally invasive pacing as a recovery procedure due to the limitation of our animal license. However, this is feasible and applicable because the minimal soft tissue injury during this procedure won’t have a great impact on animal’s health after recovery. Compared to transesophageal pacing, the only available non-invasive pacing for arium, our new method can directly pace both atrium and ventricle but requires less experience demanding ( Table 3). Secondly, unlike transvenous pacing, it is unknown whether this epicardial approach can be used for His bundle pacing despite such potential has been confirmed in humans [ 31]. Further study is needed.
5. Conclusions
The present study demonstrated a novel minimally invasive approach for assessing cardiac conduction function and arrhythmia susceptibility in mice. This new approach outplays the transvenous approach by providing similar benefits but overcoming the disadvantages by consuming less time, demanding less surgical expertise, and reducing the potential of surgical complications such as bleeding, cardiovascular and pleural ruptures, or death. Moreover, given the minimal invasiveness, the new approach has a great potential for animal recovery for longitudinal studies in drug development.
Author Contributions: Min Zi: Conceptualization, Methodology, Investigation, Writing – Original draft and revision, and Funding acquisition. Sabu Abraham: Investigation and revision. Sukhpal Prehar: Investigation and animal care. Alicia D'souza: investigation and PES protocol development. David Hutchings: PES protocol development. Xin Wang: Funding acquisition. Elizabeth J Cartwright: Funding acquisition.
Funding: This research was supported in part by BHF Seedcorn Award (P123603M2001) to M.Z., X. W. and E.J.C.
Data Availability Statement: The data underlying this article will be shared on reasonable request to the corresponding author.
Acknowledgments: We thank Nicholas Stafford for valuable comments in writing.
References
- (NICE) NIfHaCE: Atrial fibrillation: How common is it? https://cksniceorguk/topics/atrial-fibrillation/background-information/prevalence/2022.
- Colilla S.; Crow A.; Petkun W.; et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am. J. Cardiol., 2013, 112(8): 1142‒1147. DOI: https://doi.org/10.1016/j.amjcard.2013.05.063
- Go A.S.; Hylek E.M.; Phillips K.A.; et al. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA, 2001, 285(18): 2370‒2375. DOI: https://doi.org/10.1001/jama.285.18.2370
- January C.T.; Wann L.S.; Calkins H.; et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol., 2019, 74(1):104‒132. DOI: https://doi.org/10.1161/CIR.0000000000000665
- January C.T.; Wann L.S.; Alpert J.S.; et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol., 2014, 64(21):e1‒76. DOI: https://doi.org/10.1161/CIR.0000000000000041
- Wyse D.G.; Love J.C.; Yao Q.; et al. Atrial fibrillation: a risk factor for increased mortality——an AVID registry analysis. J. Interv. Card. Electrophysiol., 2001, 5(3): 267‒273.
- Anter E.; Jessup M.; Callans D.J. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation, 2009, 119(18): 2516‒2525. DOI: https://doi.org/10.1161/CIRCULATIONAHA.108.821306
- Savelieva I.; Camm A.J. Clinical relevance of silent atrial fibrillation: prevalence, prognosis, quality of life, and management. J. Interv. Card. Electrophysiol., 2000, 4(2): 369‒382.
- Bernardi J.; Aromolaran K.A.; Zhu H.; et al. Circadian mechanisms: cardiac ion channel remodeling and arrhythmias. Front. Physiol., 2021, 11: 611860. DOI: https://doi.org/10.3389/fphys.2020.611860
- Fomin D.; Chmieliauskas S.; Laima S.; et al. Sudden cardiac death in patients with coronary heart disease and antemortem alcohol intake: a STROBE - compliant retrospective study. Medicine, 2022, 101(45): e31396. DOI: https://doi.org/10.1097/MD.0000000000031396
- Koivunen M.; Tynkkynen J.; Oksala N.; et al. Incidence of sudden cardiac arrest and sudden cardiac death after unstable angina pectoris and myocardial infarction. Am. Heart J., 2023, 257: 9‒19. DOI: https://doi.org/10.1016/j.ahj.2022.11.009
- Safabakhsh S.; Al-Shaheen A.; Swiggum E.; et al. Arrhythmic sudden cardiac death in heart failure with preserved ejection fraction: mechanisms, genetics, and future directions. CJC Open, 2022, 4(11): 959‒969. DOI: https://doi.org/10.1016/j.cjco.2022.07.012
- Berul C.I.; Aronovitz M.J.; Wang P.J.; et al. In vivo cardiac electrophysiology studies in the mouse. Circulation, 1996, 94(10): 2641‒2648. DOI: https://doi.org/10.1161/01.CIR.94.10.2641
- VanderBrink B.A.; Sellitto C.; Saba S.; et al. Connexin40-deficient mice exhibit atrioventricular nodal and infra-Hisian conduction abnormalities. J. Cardiovasc. Electrophysiol., 2000, 11(11): 1270‒1276. DOI: https://doi.org/10.1046/j.1540-8167.2000.01270.x
- VanderBrink B.A.; Link M.S.; Aronovitz M.J.; et al. Assessment of atrioventricular nodal physiology in the mouse. J. Interventional Card. Electrophysiol., 1999, 3(3): 207‒212. DOI: https://doi.org/10.1023/A:1009842105146
- Schrickel J.W.; Bielik H.; Yang A.; et al. Induction of atrial fibrillation in mice by rapid transesophageal atrial pacing. Basic Res. Cardiol., 2002, 97(6): 452‒460. DOI: https://doi.org/10.1007/s003950200052
- Gutstein D.E.; Danik S.B.; Sereysky J.B.; et al. Subdiaphragmatic murine electrophysiological studies: sequential determination of ventricular refractoriness and arrhythmia induction. Am. J. Physiol.: Heart Circ. Physiol., 2003, 285(3): H1091‒H1096. DOI: https://doi.org/10.1152/ajpheart.00100.2003
- Kaese S.; Verheule S. Cardiac electrophysiology in mice: a matter of size. Front. Physiol., 2012, 3: 345. DOI: https://doi.org/10.3389/fphys.2012.00345
- Etzion Y.; Mor M.; Shalev A.; et al. New insights into the atrial electrophysiology of rodents using a novel modality: the miniature-bipolar hook electrode. Am. J. Physiol.: Heart Circ. Physiol., 2008, 295(4): H1460‒H1469. DOI: https://doi.org/10.1152/ajpheart.00414.2008
- Ripplinger C.M.; Glukhov A.V.; Kay M.W.; et al. Guidelines for assessment of cardiac electrophysiology and arrhythmias in small animals. Am. J. Physiol.: Heart Circ. Physiol., 2022, 323(6): H1137‒H1166. DOI: https://doi.org/10.1152/ajpheart.00439.2022
- Favere K.; Van Fraeyenhove J.; Jacobs G.; et al. Cardiac electrophysiology studies in mice via the transjugular route: a comprehensive practical guide. Am. J. Physiol.: Heart Circ. Physiol. 2022, 323(4): H763‒H773. DOI: https://doi.org/10.1152/ajpheart.00337.2022
- Hennis K.; Rötzer R.D.; Rilling J.; et al. In vivo and ex vivo electrophysiological study of the mouse heart to characterize the cardiac conduction system, including atrial and ventricular vulnerability. Nat. Protoc., 2022, 17(5): 1189‒1222. DOI: https://doi.org/10.1038/s41596-021-00678-z
- Atkinson W.; Forghani R.; Wojtkiewicz G.R.; et al. Ligation of the jugular veins does not result in brain inflammation or demyelination in mice. PLoS One, 2012, 7(3): e33671. DOI: https://doi.org/10.1371/journal.pone.0033671
- Chatzidou S.; Kontogiannis C.; Georgiopoulos G.; et al. Wenckebach cycle length: a novel predictor for AV block in AVNRT patients treated with ablation. Pacing Clin. Electrophysiol., 2021, 44(9): 1497‒1503. DOI: https://doi.org/10.1111/pace.14322
- Li N.; Wehrens X.H.T. Programmed electrical stimulation in mice. J. Visualized Exp., 2010, (39): 1730. DOI: https://doi.org/10.3791/1730-v
- Boukens B.J.; Rivaud M.R.; Rentschler S.; et al. Misinterpretation of the mouse ECG: ‘musing the waves of Mus musculus’. J. Physiol., 2014, 592(21): 4613‒4626. DOI: https://doi.org/10.1113/jphysiol.2014.279380
- Chowdhury S.K.; Liu W.; Zi M.; et al. Stress-activated kinase mitogen-activated kinase kinase-7 governs epigenetics of cardiac repolarization for arrhythmia prevention. Circulation, 2017, 135(7): 683‒699. DOI: https://doi.org/10.1161/CIRCULATIONAHA.116.022941
- Maguire C.T.; Wakimoto H.; Patel V.V.; et al. Implications of ventricular arrhythmia vulnerability during murine electrophysiology studies. Physiol. Genomics, 2003, 15(1): 84‒91. DOI: https://doi.org/10.1152/physiolgenomics.00034.2003
- Clasen L.; Eickholt C.; Angendohr S.; et al. A modified approach for programmed electrical stimulation in mice: inducibility of ventricular arrhythmias. PLoS One, 2018, 13(8): e0201910. DOI: https://doi.org/10.1371/journal.pone.0201910
- Wakimoto H.; Maguire C.T.; Kovoor P.; et al. Induction of atrial tachycardia and fibrillation in the mouse heart. Cardiovasc. Res., 2001, 50(3): 463‒473. DOI: https://doi.org/10.1016/S0008-6363(01)00264-4
- Kong N.W.; Upadhyay G.A. Cardiac resynchronization considerations in left bundle branch block. Front. Physiol., 2022, 13: 962042. DOI: https://doi.org/10.3389/fphys.2022.962042






