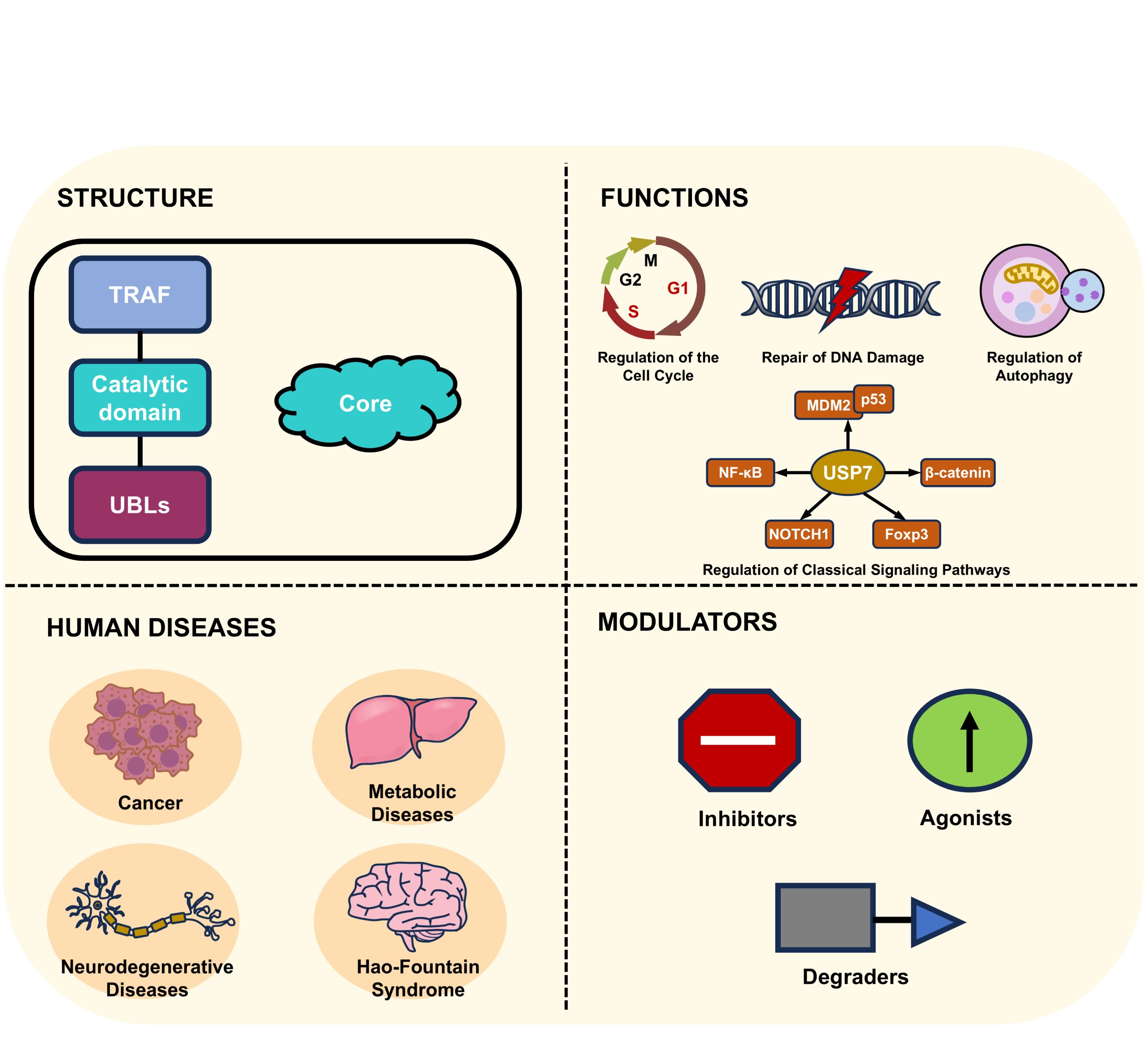
Ubiquitin-specific protease 7 (USP7) is a crucial member of the deubiquitinase family. USP7 exhibits unique structural characteristics, consisting of an N-terminal TRAF domain, a catalytic domain, and C-terminal ubiquitin-like (UBL) domains. Notably, the dynamic switch between inactive and active conformations in the catalytic domain confers precise control of its enzymatic activity. USP7 plays pivotal roles in cell cycle progression, DNA damage repair, and key signaling pathways through deubiquitinating critical regulatory factors. Dysregulation of USP7 triggers various diseases, including cancers, metabolic disorders, neurodegenerative diseases, and Hao-Fountain syndrome. This review systematically summarizes structural features and physiological functions of USP7, and elucidates its regulatory mechanisms in disease pathogenesis. Additionally, currently reported USP7 targeted modulators, including inhibitors, agonists, and degraders, are also summarized. These insights provide theoretical foundations for developing novel regulators and potential therapeutic strategies for related diseases.
- Open Access
- Review
Structural Plasticity Guides Functional Versatility of USP7 in Human Diseases: Mechanistic Insights and Therapeutic Targeting
- Xuanyu Xu 1, 2,
- Naixia Zhang 1, 2,
- Li Shi 1, *
Author Information
Received: 30 May 2025 | Revised: 19 Jun 2025 | Accepted: 10 Jul 2025 | Published: 15 Aug 2025
Abstract
Graphical Abstract
Keywords
References
- 1.Popovic, D.; Vucic, D.; Dikic, I. Ubiquitination in Disease Pathogenesis and Treatment. Nat. Med. 2014, 20, 1242–1253. https://doi.org/10.1038/nm.3739.
- 2.Lacoursiere, R.E.; Hadi, D.; Shaw, G.S. Acetylation, Phosphorylation, Ubiquitination (Oh My!): Following Post-Translational Modifications on the Ubiquitin Road. Biomolecules 2022, 12, 467. https://doi.org/10.3390/biom12030467.
- 3.Fraile, J.M.; Quesada, V.; Rodríguez, D.; Freije, J.M.P.; López-Otín, C. Deubiquitinases in Cancer: New Functions and Therapeutic Options. Oncogene 2012, 31, 2373–2388. https://doi.org/10.1038/onc.2011.443.
- 4.Kim, Y.; Kim, E.K.; Chey, Y.; Song, M.J.; Jang, H.H. Targeted Protein Degradation: Principles and Applications of the Proteasome. Cells 2023, 12, 1846. https://doi.org/10.3390/cells12141846.
- 5.Chen, S.; Liu, Y.; Zhou, H. Advances in the Development Ubiquitin-Specific Peptidase (USP) Inhibitors. Int. J. Mol. Sci. 2021, 22, 4546. https://doi.org/10.3390/ijms22094546.
- 6.Everett, R.D.; Meredith, M.; Orr, A.; Cross, A.; Kathoria, M.; Parkinson, J. A Novel Ubiquitin-Specific Protease Is Dynamically Associated with the PML Nuclear Domain and Binds to a Herpesvirus Regulatory Protein. EMBO J. 1997, 16, 566–577. https://doi.org/10.1093/emboj/16.3.566.
- 7.Bhattacharya, S.; Chakraborty, D.; Basu, M.; Ghosh, M.K. Emerging Insights into HAUSP (USP7) in Physiology, Cancer and Other Diseases. Signal Transduct. Target. Ther. 2018, 3, 17. https://doi.org/10.1038/s41392-018-0012-y.
- 8.Granieri, L.; Marocchi, F.; Melixetian, M.; Mohammadi, N.; Nicoli, P.; Cuomo, A.; Bonaldi, T.; Confalonieri, S.; Pisati, F.; Giardina, G.; et al. Targeting the USP7/RRM2 Axis Drives Senescence and Sensitizes Melanoma Cells to HDAC/LSD1 Inhibitors. Cell Rep. 2022, 40, 111396. https://doi.org/10.1016/j.celrep.2022.111396.
- 9.Liu, X.; Lu, R.; Yang, Q.; He, J.; Huang, C.; Cao, Y.; Zhou, Z.; Huang, J.; Li, L.; Chen, R.; et al. USP7 Reduces the Level of Nuclear DICER, Impairing DNA Damage Response and Promoting Cancer Progression. Mol. Oncol. 2024, 18, 170–189. https://doi.org/10.1002/1878-0261.13543.
- 10.Tavana, O.; Gu, W. Modulation of the P53/MDM2 Interplay by HAUSP Inhibitors. J. Mol. Cell Biol. 2017, 9, 45–52. https://doi.org/10.1093/jmcb/mjw049.
- 11.Ji, L.; Lu, B.; Zamponi, R.; Charlat, O.; Aversa, R.; Yang, Z.; Sigoillot, F.; Zhu, X.; Hu, T.; Reece-Hoyes, J.S.; et al. USP7 Inhibits Wnt/β-Catenin Signaling through Promoting Stabilization of Axin. Nat. Commun. 2019, 10, 4184. https://doi.org/10.1038/s41467-019-12143-3.
- 12.Franqui-Machin, R.; Hao, M.; Bai, H.; Gu, Z.; Zhan, X.; Habelhah, H.; Jethava, Y.; Qiu, L.; Frech, I.; Tricot, G.; et al. Destabilizing NEK2 Overcomes Resistance to Proteasome Inhibition in Multiple Myeloma. J. Clin. Investig. 2018, 128, 2877–2893. https://doi.org/10.1172/JCI98765.
- 13.Saha, G.; Roy, S.; Basu, M.; Ghosh, M.K. USP7—A Crucial Regulator of Cancer Hallmarks. Biochim. Biophys. Acta BBA—Rev. Cancer 2023, 1878, 188903. https://doi.org/10.1016/j.bbcan.2023.188903.
- 14.Birks, E.J.; Latif, N.; Enesa, K.; Folkvang, T.; Luong, L.A.; Sarathchandra, P.; Khan, M.; Ovaa, H.; Terracciano, C.M.; Barton, P.J.R.; et al. Elevated P53 Expression Is Associated with Dysregulation of the Ubiquitin-Proteasome System in Dilated Cardiomyopathy. Cardiovasc. Res. 2008, 79, 472–480. https://doi.org/10.1093/cvr/cvn083.
- 15.Zhang, X.W.; Feng, N.; Liu, Y.C.; Guo, Q.; Wang, J.K.; Bai, Y.Z.; Ye, X.M.; Yang, Z.; Yang, H.; Liu, Y.; et al. Neuroinflammation inhibition by small-molecule targeting USP7 noncatalytic domain for neurodegenerative disease therapy. Sci. Adv. 2022, 8, eabo0789. https://doi.org/10.1126/sciadv.abo0789.
- 16.Hao, Y.H.; Fountain, M.D.; Fon Tacer, K.; Xia, F.; Bi, W.; Kang, S.H.; Patel, A.; Rosenfeld, J.A.; Le Caignec, C.; Isidor, B.; et al. USP7 Acts as a Molecular Rheostat to Promote WASH-Dependent Endosomal Protein Recycling and Is Mutated in a Human Neurodevelopmental Disorder. Mol. Cell. 2015, 59, 956–969. https://doi.org/10.1016/j.molcel.2015.07.033.
- 17.Oliveira, R.I.; Guedes, R.A.; Salvador, J.A.R. Highlights in USP7 Inhibitors for Cancer Treatment. Front. Chem. 2022, 10, 1005727. https://doi.org/10.3389/fchem.2022.1005727.
- 18.Shi, L.; Xu, Z.; Chen, X.; Meng, Q.; Zhou, H.; Xiong, B.; Zhang, N. Sertraline and Astemizole Enhance the Deubiquitinase Activity of USP7 by Binding to Its Switching Loop Region. J. Med. Chem. 2025, 68, 5874–5890. https://doi.org/10.1021/acs.jmedchem.5c00032.
- 19.Maisonet, I.J.; Sharafi, M.; Korchak, E.J.; Salazar-Chaparro, A.; Bratt, A.; Parikh, T.; Varca, A.C.; Shah, B.; Darnowski, M.; Chung, M.; et al. Small-Molecule Allosteric Activator of Ubiquitin-Specific Protease 7 (USP7). bioRxiv 2025, 2025, 643379. https://doi.org/10.1101/2025.03.14.643379.
- 20.Saridakis, V.; Sheng, Y.; Sarkari, F.; Holowaty, M.N.; Shire, K.; Nguyen, T.; Zhang, R.G.; Liao, J.; Lee, W.; Edwards, A.M.; et al. Structure of the P53 Binding Domain of HAUSP/USP7 Bound to Epstein-Barr Nuclear Antigen 1. Mol. Cell. 2005, 18, 25–36. https://doi.org/10.1016/j.molcel.2005.02.029.
- 21.Pozhidaeva, A.; Bezsonova, I. USP7: Structure, Substrate Specificity, and Inhibition. DNA Repair 2019, 76, 30–39. https://doi.org/10.1016/j.dnarep.2019.02.005.
- 22.Harakandi, C.; Nininahazwe, L.; Xu, H.; Liu, B.; He, C.; Zheng, Y.C.; Zhang, H. Recent Advances on the Intervention Sites Targeting USP7-MDM2-P53 in Cancer Therapy. Bioorg. Chem. 2021, 116, 105273. https://doi.org/10.1016/j.bioorg.2021.105273.
- 23.Hu, M.; Li, P.; Li, M.; Li, W.; Yao, T.; Wu, J.W.; Gu, W.; Cohen, R.E.; Shi, Y. Crystal Structure of a UBP-Family Deubiquitinating Enzyme in Isolation and in Complex with Ubiquitin Aldehyde. Cell 2002, 111, 1041–1054. https://doi.org/10.1016/s0092-8674(02)01199-6.
- 24.Nininahazwe, L.; Liu, B.; He, C.; Zhang, H.; Chen, Z.S. The Emerging Nature of Ubiquitin-Specific Protease 7 (USP7): A New Target in Cancer Therapy. Drug Discov. Today. 2021, 26, 490–502. https://doi.org/10.1016/j.drudis.2020.10.028.
- 25.Guo, N.J.; Wang, B.; Zhang, Y.; Kang, H.Q.; Nie, H.Q.; Feng, M.K.; Zhang, X.Y.; Zhao, L.J.; Wang, N.; Liu, H.M.; et al. USP7 as an Emerging Therapeutic Target: A Key Regulator of Protein Homeostasis. Int. J. Biol. Macromol. 2024, 263, 130309. https://doi.org/10.1016/j.ijbiomac.2024.130309.
- 26.Rougé, L.; Bainbridge, T.W.; Kwok, M.; Tong, R.; Di Lello, P.; Wertz, I.E.; Maurer, T.; Ernst, J.A.; Murray, J. Molecular Understanding of USP7 Substrate Recognition and C-Terminal Activation. Structure 2016, 24, 1335–1345. https://doi.org/10.1016/j.str.2016.05.020.
- 27.Holowaty, M.N.; Sheng, Y.; Nguyen, T.; Arrowsmith, C.; Frappier, L. Protein Interaction Domains of the Ubiquitin-Specific Protease, USP7/HAUSP. J. Biol. Chem. 2003, 278, 47753–47761. https://doi.org/10.1074/jbc.M307200200.
- 28.Zhang, Z.M.; Rothbart, S.B.; Allison, D.F.; Cai, Q.; Harrison, J.S.; Li, L.; Wang, Y.; Strahl, B.D.; Wang, G.G.; Song, J. An Allosteric Interaction Links USP7 to Deubiquitination and Chromatin Targeting of UHRF1. Cell Rep. 2015, 12, 1400–1406. https://doi.org/10.1016/j.celrep.2015.07.046.
- 29.Cheng, J.; Yang, H.; Fang, J.; Ma, L.; Gong, R.; Wang, P.; Li, Z.; Xu, Y. Molecular Mechanism for USP7-Mediated DNMT1 Stabilization by Acetylation. Nat. Commun. 2015, 6, 7023. https://doi.org/10.1038/ncomms8023.
- 30.van der Horst, A.; de Vries-Smits, A.M.M.; Brenkman, A.B.; van Triest, M.H.; van den Broek, N.; Colland, F.; Maurice, M.M.; Burgering, B.M.T. FOXO4 Transcriptional Activity Is Regulated by Monoubiquitination and USP7/HAUSP. Nat. Cell Biol. 2006, 8, 1064–1073. https://doi.org/10.1038/ncb1469.
- 31.Faesen, A.C.; Dirac, A.M.; Shanmugham, A.; Ovaa, H.; Perrakis, A.; Sixma, T.K. Mechanism of USP7/HAUSP Activation by Its C-Terminal Ubiquitin-like Domain and Allosteric Regulation by GMP-Synthetase. Mol. Cell. 2011, 44, 147–159. https://doi.org/10.1016/j.molcel.2011.06.034.
- 32.Jenkins, Y.; Markovtsov, V.; Lang, W.; Sharma, P.; Pearsall, D.; Warner, J.; Franci, C.; Huang, B.; Huang, J.; Yam, G.C.; et al. Critical Role of the Ubiquitin Ligase Activity of UHRF1, a Nuclear RING Finger Protein, in Tumor Cell Growth. Mol. Biol. Cell. 2005, 16, 5621–5629. https://doi.org/10.1091/mbc.e05-03-0194.
- 33.Qin, W.; Wolf, P.; Liu, N.; Link, S.; Smets, M.; Mastra, F.L.; Forné, I.; Pichler, G.; Hörl, D.; Fellinger, K.; et al. DNA Methylation Requires a DNMT1 Ubiquitin Interacting Motif (UIM) and Histone Ubiquitination. Cell Res. 2015, 25, 911–929. https://doi.org/10.1038/cr.2015.72.
- 34.Qin, W.; Leonhardt, H.; Spada, F. Usp7 and Uhrf1 Control Ubiquitination and Stability of the Maintenance DNA Methyltransferase Dnmt1. J. Cell. Biochem. 2011, 112, 439–444. https://doi.org/10.1002/jcb.22998.
- 35.Li, J.; Wang, R.; Jin, J.; Han, M.; Chen, Z.; Gao, Y.; Hu, X.; Zhu, H.; Gao, H.; Lu, K.; et al. USP7 Negatively Controls Global DNA Methylation by Attenuating Ubiquitinated Histone-Dependent DNMT1 Recruitment. Cell Discov. 2020, 6, 58. https://doi.org/10.1038/s41421-020-00188-4.
- 36.Sharma, S.S.; Pledger, W.J.; Kondaiah, P. The Deubiquitylase USP7 Is a Novel Cyclin F-Interacting Protein and Regulates Cyclin F Protein Stability. Aging 2022, 14, 8645–8660. https://doi.org/10.18632/aging.204372.
- 37.Galarreta, A.; Valledor, P.; Ubieto-Capella, P.; Lafarga, V.; Zarzuela, E.; Muñoz, J.; Malumbres, M.; Lecona, E.; Fernandez-Capetillo, O. USP7 Limits CDK1 Activity throughout the Cell Cycle. EMBO J. 2021, 40, e99692. https://doi.org/10.15252/embj.201899692.
- 38.Jackson, S.P.; Bartek, J. The DNA-Damage Response in Human Biology and Disease. Nature 2009, 461, 1071–1078. https://doi.org/10.1038/nature08467.
- 39.Wang, R.; Sun, Y.; Li, C.; Xue, Y.; Ba, X. Targeting the DNA Damage Response for Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 15907. https://doi.org/10.3390/ijms242115907.
- 40.Liu, J.; Zhou, T.; Dong, X.; Guo, Q.; Zheng, L.; Wang, X.; Zhang, N.; Li, D.; Ren, L.; Yi, F.; et al. De-Ubiquitination of SAMHD1 by USP7 Promotes DNA Damage Repair to Overcome Oncogenic Stress and Affect Chemotherapy Sensitivity. Oncogene 2023, 42, 1843–1856. https://doi.org/10.1038/s41388-023-02667-w.
- 41.Daddacha, W.; Koyen, A.E.; Bastien, A.J.; Head, P.E.; Dhere, V.R.; Nabeta, G.N.; Connolly, E.C.; Werner, E.; Madden, M.Z.; Daly, M.B.; et al. SAMHD1 Promotes DNA End Resection to Facilitate DNA Repair by Homologous Recombination. Cell Rep. 2017, 20, 1921–1935. https://doi.org/10.1016/j.celrep.2017.08.008.
- 42.Lu, H.; Shamanna, R.A.; de Freitas, J.K.; Okur, M.; Khadka, P.; Kulikowicz, T.; Holland, P.P.; Tian, J.; Croteau, D.L.; Davis, A.J.; et al. Cell Cycle-Dependent Phosphorylation Regulates RECQL4 Pathway Choice and Ubiquitination in DNA Double-Strand Break Repair. Nat. Commun. 2017, 8, 2039. https://doi.org/10.1038/s41467-017-02146-3.
- 43.Huang, Q.; Qin, D.; Pei, D.; Vermeulen, M.; Zhang, X. UBE2O and USP7 Co-Regulate RECQL4 Ubiquitinylation and Homologous Recombination-Mediated DNA Repair. FASEB J. 2022, 36, e22112. https://doi.org/10.1096/fj.202100974RRR.
- 44.Lin, N.Y.; Chen, C.W.; Kagwiria, R.; Liang, R.; Beyer, C.; Distler, A.; Luther, J.; Engelke, K.; Schett, G.; Distler, J.H. Inactivation of Autophagy Ameliorates Glucocorticoid-Induced and Ovariectomy-Induced Bone Loss. Ann. Rheum. Dis. 2016, 75, 1203–1210. https://doi.org/10.1136/annrheumdis-2015-207240.
- 45.Klionsky, D.J.; Petroni, G.; Amaravadi, R.K.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cadwell, K.; Cecconi, F.; Choi, A.M.K.; et al. Autophagy in Major Human Diseases. EMBO J. 2021, 40, e108863. https://doi.org/10.15252/embj.2021108863.
- 46.Pluciennik, A.; Liu, Y.; Molotsky, E.; Marsh, G.B.; Ranxhi, B.; Arnold, F.J.; St. Cyr, S.; Davidson, B.; Pourshafie, N.; Lieberman, A.P.; et al. Deubiquitinase USP7 Contributes to the Pathogenicity of Spinal and Bulbar Muscular Atrophy. J. Clin. Investig. 2021, 131, e134565. https://doi.org/10.1172/JCI134565.
- 47.Lu, X.; Zhang, Y.; Zheng, Y.; Chen, B. The miRNA-15b/USP7/KDM6B Axis Engages in the Initiation of Osteoporosis by Modulating Osteoblast Differentiation and Autophagy. J. Cell. Mol. Med. 2021, 25, 2069–2081. https://doi.org/10.1111/jcmm.16139.
- 48.Keshri, S.; Vicinanza, M.; Takla, M.; Rubinsztein, D.C. USP7 Protects TFEB from Proteasome-Mediated degradationUSP7. Cell Rep. 2024, 43, 114872. https://doi.org/10.1016/j.celrep.2024.114872.
- 49.Reed, S.M.; Quelle, D.E. P53 Acetylation: Regulation and Consequences. Cancers 2015, 7, 30–69. https://doi.org/10.3390/cancers7010030.
- 50.Lee, J.T.; Gu, W. The Multiple Levels of Regulation by P53 Ubiquitination. Cell Death Differ. 2010, 17, 86–92. https://doi.org/10.1038/cdd.2009.77.
- 51.Sheng, Y.; Saridakis, V.; Sarkari, F.; Duan, S.; Wu, T.; Arrowsmith, C.H.; Frappier, L. Molecular Recognition of P53 and MDM2 by USP7/HAUSP. Nat. Struct. Mol. Biol. 2006, 13, 285–291. https://doi.org/10.1038/nsmb1067.
- 52.Bonacci, T.; Emanuele, M.J. Dissenting Degradation: Deubiquitinases in Cell Cycle and Cancer. Semin. Cancer Biol. 2020, 67, 145–158. https://doi.org/10.1016/j.semcancer.2020.03.008.
- 53.Qi, S.M.; Cheng, G.; Cheng, X.D.; Xu, Z.; Xu, B.; Zhang, W.D.; Qin, J.J. Targeting USP7-Mediated Deubiquitination of MDM2/MDMX-P53 Pathway for Cancer Therapy: Are We There Yet? Front. Cell Dev. Biol. 2020, 8, 233. https://doi.org/10.3389/fcell.2020.00233.
- 54.Kwon, S.K.; Saindane, M.; Baek, K.H. P53 Stability Is Regulated by Diverse Deubiquitinating Enzymes. Biochim. Biophys. Acta BBA—Rev. Cancer 2017, 1868, 404–411. https://doi.org/10.1016/j.bbcan.2017.08.001.
- 55.Rawat, R.; Starczynowski, D.T.; Ntziachristos, P. Nuclear Deubiquitination in the Spotlight: The Multifaceted Nature of USP7 Biology in Disease. Curr. Opin. Cell Biol. 2019, 58, 85–94. https://doi.org/10.1016/j.ceb.2019.02.008.
- 56.Aberle, H.; Bauer, A.; Stappert, J.; Kispert, A.; Kemler, R. Beta-catenin Is a Target for the Ubiquitin–Proteasome Pathway. EMBO J. 1997, 16, 3797–3804. https://doi.org/10.1093/emboj/16.13.3797.
- 57.An, T.; Gong, Y.; Li, X.; Kong, L.; Ma, P.; Gong, L.; Zhu, H.; Yu, C.; Liu, J.; Zhou, H.; et al. USP7 Inhibitor P5091 Inhibits Wnt Signaling and Colorectal Tumor Growth. Biochem. Pharmacol. 2017, 131, 29–39. https://doi.org/10.1016/j.bcp.2017.02.011.
- 58.Novellasdemunt, L.; Foglizzo, V.; Cuadrado, L.; Antas, P.; Kucharska, A.; Encheva, V.; Snijders, A.P.; Li, V.S.W. USP7 Is a Tumor-Specific WNT Activator for APC-Mutated Colorectal Cancer by Mediating β-Catenin Deubiquitination. Cell Rep. 2017, 21, 612–627. https://doi.org/10.1016/j.celrep.2017.09.072.
- 59.Novellasdemunt, L.; Kucharska, A.; Baulies, A.; Hutton, C.; Vlachogiannis, G.; Repana, D.; Rowan, A.; Suárez-Bonnet, A.; Ciccarelli, F.; Valeri, N.; et al. USP7 Inactivation Suppresses APC-Mutant Intestinal Hyperproliferation and Tumor Development. Stem Cell Rep. 2023, 18, 570–584. https://doi.org/10.1016/j.stemcr.2022.12.013.
- 60.Zhang, F.; Zhang, B.; Tang, R.; Jiang, H.; Ji, Z.; Chen, Y.; Feng, H. The Occurrence of Lupus Nephritis Is Regulated by USP7-Mediated JMJD3 Stabilization. Immunol. Lett. 2021, 235, 41–50. https://doi.org/10.1016/j.imlet.2021.04.006.
- 61.Colleran, A.; Collins, P.E.; O’Carroll, C.; Ahmed, A.; Mao, X.; McManus, B.; Kiely, P.A.; Burstein, E.; Carmody, R.J. Deubiquitination of NF-κB by Ubiquitin-Specific Protease-7 Promotes Transcription. Proc. Natl. Acad. Sci. USA 2013, 110, 618–623. https://doi.org/10.1073/pnas.1208446110.
- 62.Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB Pathway for the Therapy of Diseases: Mechanism and Clinical Study. Signal Transduct. Target. Ther. 2020, 5, 1–23. https://doi.org/10.1038/s41392-020-00312-6.
- 63.Yao, Y.; Zhang, Y.; Shi, M.; Sun, Y.; Chen, C.; Niu, M.; Zhang, Q.; Zeng, L.; Yao, R.; Li, H.; et al. Blockade of Deubiquitinase USP7 Overcomes Bortezomib Resistance by Suppressing NF-κB Signaling Pathway in Multiple Myeloma. J. Leukoc. Biol. 2018, 104, 1105–1115. https://doi.org/10.1002/JLB.2A1017-420RR.
- 64.Ye, M.; He, J.; Zhang, J.; Liu, B.; Liu, X.; Xie, L.; Wei, M.; Dong, R.; Li, K.; Ma, D.; et al. USP7 Promotes Hepatoblastoma Progression through Activation of PI3K/AKT Signaling Pathway. Cancer Biomark. 2021, 31, 107–117. https://doi.org/10.3233/CBM-200052.
- 65.Weng, A.P.; Ferrando, A.A.; Lee, W.; Morris, J.P.; Silverman, L.B.; Sanchez-Irizarry, C.; Blacklow, S.C.; Look, A.T.; Aster, J.C. Activating Mutations of NOTCH1 in Human T Cell Acute Lymphoblastic Leukemia. Science 2004, 306, 269–271. https://doi.org/10.1126/science.1102160.
- 66.Shan, H.; Li, X.; Xiao, X.; Dai, Y.; Huang, J.; Song, J.; Liu, M.; Yang, L.; Lei, H.; Tong, Y.; et al. USP7 Deubiquitinates and Stabilizes NOTCH1 in T-Cell Acute Lymphoblastic Leukemia. Signal Transduct. Target. Ther. 2018, 3, 1–10. https://doi.org/10.1038/s41392-018-0028-3.
- 67.Jin, Q.; Martinez, C.A.; Arcipowski, K.M.; Zhu, Y.; Gutierrez-Diaz, B.T.; Wang, K.K.; Johnson, M.R.; Volk, A.G.; Wang, F.; Wu, J.; et al. USP7 Cooperates with NOTCH1 to Drive the Oncogenic Transcriptional Program in T-Cell Leukemia. Clin. Cancer Res. 2019, 25, 222–239. https://doi.org/10.1158/1078-0432.CCR-18-1740.
- 68.van Loosdregt, J.; Fleskens, V.; Fu, J.; Brenkman, A.B.; Bekker, C.P.J.; Pals, C.E.G.M.; Meerding, J.; Berkers, C.R.; Barbi, J.; Gröne, A.; et al. Stabilization of the Transcription Factor Foxp3 by the Deubiquitinase USP7 Increases Treg-Cell-Suppressive Capacity. Immunity 2013, 39, 259–271. https://doi.org/10.1016/j.immuni.2013.05.018.
- 69.Wang, L.; Kumar, S.; Dahiya, S.; Wang, F.; Wu, J.; Newick, K.; Han, R.; Samanta, A.; Beier, U.H.; Akimova, T.; et al. Ubiquitin-Specific Protease-7 Inhibition Impairs Tip60-Dependent Foxp3 + T-Regulatory Cell Function and Promotes Antitumor Immunity. EBioMedicine 2016, 13, 99–112. https://doi.org/10.1016/j.ebiom.2016.10.018.
- 70.Colombino, M.; Paliogiannis, P.; Cossu, A.; Santeufemia, D.A.; Pazzola, A.; Fadda, G.M.; Pirina, P.; Fois, A.; Putzu, C.; Ginesu, G.; et al. EGFR, KRAS, BRAF, ALK, and cMET Genetic Alterations in 1440 Sardinian Patients with Lung Adenocarcinoma. BMC Pulm. Med. 2019, 19, 209. https://doi.org/10.1186/s12890-019-0964-x.
- 71.Pylayeva-Gupta, Y.; Grabocka, E.; Bar-Sagi, D. RAS Oncogenes: Weaving a Tumorigenic Web. Nat. Rev. Cancer 2011, 11, 761–774. https://doi.org/10.1038/nrc3106.
- 72.Huang, B.; Cao, D.; Yuan, X.; Xiong, Y.; Chen, B.; Wang, Y.; Niu, X.; Tian, R.; Huang, H. USP7 Deubiquitinates KRAS and Promotes Non-Small Cell Lung Cancer. Cell Rep. 2024, 43, 114917. https://doi.org/10.1016/j.celrep.2024.114917.
- 73.Dai, X.; Lu, L.; Deng, S.; Meng, J.; Wan, C.; Huang, J.; Sun, Y.; Hu, Y.; Wu, B.; Wu, G.; et al. USP7 Targeting Modulates Anti-Tumor Immune Response by Reprogramming Tumor-Associated Macrophages in Lung Cancer. Theranostics 2020, 10, 9332–9347. https://doi.org/10.7150/thno.47137.
- 74.Hu, H.; Zhao, K.; Fang, D.; Wang, Z.; Yu, N.; Yao, B.; Liu, K.; Wang, F.; Mei, Y. The RNA Binding Protein RALY Suppresses P53 Activity and Promotes Lung Tumorigenesis. Cell Rep. 2023, 42, 112288. https://doi.org/10.1016/j.celrep.2023.112288.
- 75.Chen, S.T.; Okada, M.; Nakato, R.; Izumi, K.; Bando, M.; Shirahige, K. The Deubiquitinating Enzyme USP7 Regulates Androgen Receptor Activity by Modulating Its Binding to Chromatin. J. Biol. Chem. 2015, 290, 21713–21723. https://doi.org/10.1074/jbc.M114.628255.
- 76.Morra, F.; Merolla, F.; Napolitano, V.; Ilardi, G.; Miro, C.; Paladino, S.; Staibano, S.; Cerrato, A.; Celetti, A. The Combined Effect of USP7 Inhibitors and PARP Inhibitors in Hormone-Sensitive and Castration-Resistant Prostate Cancer Cells. Oncotarget 2017, 8, 31815–31829. https://doi.org/10.18632/oncotarget.16463.
- 77.Gao, N.; Ishii, K.; Mirosevich, J.; Kuwajima, S.; Oppenheimer, S.R.; Roberts, R.L.; Jiang, M.; Yu, X.; Shappell, S.B.; Caprioli, R.M.; et al. Forkhead Box A1 Regulates Prostate Ductal Morphogenesis and Promotes Epithelial Cell Maturation. Development 2005, 132, 3431–3443. https://doi.org/10.1242/dev.01917.
- 78.Xu, B.; Song, B.; Lu, X.; Kim, J.; Hu, M.; Zhao, J.C.; Yu, J. Altered Chromatin Recruitment by FOXA1 Mutations Promotes Androgen Independence and Prostate Cancer Progression. Cell Res. 2019, 29, 773–775. https://doi.org/10.1038/s41422-019-0204-1.
- 79.Park, S.H.; Fong, K.; Kim, J.; Wang, F.; Lu, X.; Lee, Y.; Brea, L.T.; Wadosky, K.; Guo, C.; Abdulkadir, S.A.; et al. Posttranslational Regulation of FOXA1 by Polycomb and BUB3/USP7 Deubiquitin Complex in Prostate Cancer. Sci. Adv. 2021, 7, eabe2261. https://doi.org/10.1126/sciadv.abe2261.
- 80.Ersvær, E.; Kildal, W.; Vlatkovic, L.; Cyll, K.; Pradhan, M.; Kleppe, A.; Hveem, T.S.; Askautrud, H.A.; Novelli, M.; Wæhre, H.; et al. Prognostic Value of Mitotic Checkpoint Protein BUB3, Cyclin B1, and Pituitary Tumor-Transforming 1 Expression in Prostate Cancer. Mod. Pathol. 2020, 33, 905–915. https://doi.org/10.1038/s41379-019-0418-2.
- 81.Song, M.S.; Salmena, L.; Carracedo, A.; Egia, A.; Lo-Coco, F.; Teruya-Feldstein, J.; Pandolfi, P.P. The Deubiquitinylation and Localization of PTEN Are Regulated by a HAUSP–PML Network. Nature 2008, 455, 813–817. https://doi.org/10.1038/nature07290.
- 82.Zhang, Q.; Cao, C.; Gong, W.; Bao, K.; Wang, Q.; Wang, Y.; Bi, L.; Ma, S.; Zhao, J.; Liu, L.; et al. A Feedforward Circuit Shaped by ECT2 and USP7 Contributes to Breast Carcinogenesis. Theranostics 2020, 10, 10769–10790. https://doi.org/10.7150/thno.46878.
- 83.He, J.; Li, C.F.; Lee, H.J.; Shin, D.H.; Chern, Y.J.; Carvalho, B.P.D.; Chan, C.H. MIG-6 Is Essential for Promoting Glucose Metabolic Reprogramming and Tumor Growth in Triple-negative Breast Cancer. EMBO Rep. 2021, 22, e50781. https://doi.org/10.15252/embr.202050781.
- 84.Yi, J.; Li, H.; Chu, B.; Kon, N.; Hu, X.; Hu, J.; Xiong, Y.; Kaniskan, H.U.; Jin, J.; Gu, W. Inhibition of USP7 Induces P53-Independent Tumor Growth Suppression in Triple-Negative Breast Cancers by Destabilizing FOXM1. Cell Death Differ. 2023, 30, 1799–1810. https://doi.org/10.1038/s41418-023-01180-7.
- 85.Zhu, Y.; Gu, L.; Lin, X.; Cui, K.; Liu, C.; Lu, B.; Zhou, F.; Zhao, Q.; Shen, H.; Li, Y. LINC00265 Promotes Colorectal Tumorigenesis via ZMIZ2 and USP7-Mediated Stabilization of β-Catenin. Cell Death Differ. 2020, 27, 1316–1327. https://doi.org/10.1038/s41418-019-0417-3.
- 86.Jiang, L.; Xiong, J.; Zhan, J.; Yuan, F.; Tang, M.; Zhang, C.; Cao, Z.; Chen, Y.; Lu, X.; Li, Y.; et al. Ubiquitin-Specific Peptidase 7 (USP7)-Mediated Deubiquitination of the Histone Deacetylase SIRT7 Regulates Gluconeogenesis. J. Biol. Chem. 2017, 292, 13296–13311. https://doi.org/10.1074/jbc.M117.780130.
- 87.Yan, M.; Su, L.; Wu, K.; Mei, Y.; Liu, Z.; Chen, Y.; Zeng, W.; Xiao, Y.; Zhang, J.; Cai, G.; et al. USP7 Promotes Cardiometabolic Disorders and Mitochondrial Homeostasis Dysfunction in Diabetic Mice via Stabilizing PGC1β. Pharmacol. Res. 2024, 205, 107235. https://doi.org/10.1016/j.phrs.2024.107235.
- 88.Ni, W.; Lin, S.; Bian, S.; Zheng, W.; Qu, L.; Fan, Y.; Lu, C.; Xiao, M.; Zhou, P. USP7 Mediates Pathological Hepatic de Novo Lipogenesis through Promoting Stabilization and Transcription of ZNF638. Cell Death Dis. 2020, 11, 1–17. https://doi.org/10.1038/s41419-020-03075-8.
- 89.Zhang, Y.; Zhang, Y. Knockdown of USP7 Alleviates Atherosclerosis in ApoE-Deficient Mice by Regulating EZH2 Expression. Open Life Sci. 2024, 19, 20220929. https://doi.org/10.1515/biol-2022-0929.
- 90.Prusiner, S.B. A Unifying Role for Prions in Neurodegenerative Diseases. Science 2012, 336, 1511–1513. https://doi.org/10.1126/science.1222951.
- 91.Wilson, D.M.; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of Neurodegenerative Diseases. Cell 2023, 186, 693–714. https://doi.org/10.1016/j.cell.2022.12.032.
- 92.Zhang, T.; Periz, G.; Lu, Y.N.; Wang, J. USP7 Regulates ALS-Associated Proteotoxicity and Quality Control through the NEDD4L–SMAD Pathway. Proc. Natl. Acad. Sci. USA 2020, 117, 28114–28125. https://doi.org/10.1073/pnas.2014349117.
- 93.Kim, J.; de Haro, M.; Al-Ramahi, I.; Garaicoechea, L.L.; Jeong, H.H.; Sonn, J.Y.; Tadros, B.; Liu, Z.; Botas, J.; Zoghbi, H.Y. Evolutionarily Conserved Regulators of Tau Identify Targets for New Therapies. Neuron 2023, 111, 824–838.e7. https://doi.org/10.1016/j.neuron.2022.12.012.
- 94.Hao, Y.H.; Doyle, J.M.; Ramanathan, S.; Gomez, T.S.; Jia, D.; Xu, M.; Chen, Z.J.; Billadeau, D.D.; Rosen, M.K.; Potts, P.R. Regulation of WASH-Dependent Actin Polymerization and Protein Trafficking by Ubiquitination. Cell 2013, 152, 1051–1064. https://doi.org/10.1016/j.cell.2013.01.051.
- 95.Fountain, M.D.; Oleson, D.S.; Rech, M.E.; Segebrecht, L.; Hunter, J.V.; McCarthy, J.M.; Lupo, P.J.; Holtgrewe, M.; Moran, R.; Rosenfeld, J.A.; et al. Pathogenic Variants in USP7 Cause a Neurodevelopmental Disorder with Speech Delays, Altered Behavior, and Neurologic Anomalies. Genet. Med. 2019, 21, 1797–1807. https://doi.org/10.1038/s41436-019-0433-1.
- 96.Zampieri, N.; Pulvirenti, R.; Pedrazzoli, E.; Camoglio, F.S. Hao-Fountain Syndrome and Genital Disorders: Report of a New Possible Association. Ital. J. Pediatr. 2022, 48, 182. https://doi.org/10.1186/s13052-022-01367-7.
- 97.Capra, A.P.; Agolini, E.; La Rosa, M.A.; Novelli, A.; Briuglia, S. Correspondence on “Pathogenic Variants in USP7 Cause a Neurodevelopmental Disorder with Speech Delays, Altered Behavior, and Neurologic Anomalies” by Fountain et al. Genet. Med. 2021, 23, 421–422. https://doi.org/10.1038/s41436-020-00978-x.
- 98.van der Laan, L.; Karimi, K.; Rooney, K.; Lauffer, P.; McConkey, H.; Caro, P.; Relator, R.; Levy, M.A.; Bhai, P.; Mignot, C.; et al. DNA Methylation Episignature, Extension of the Clinical Features, and Comparative Epigenomic Profiling of Hao-Fountain Syndrome Caused by Variants in USP7. Genet. Med. 2024, 26, 101050. https://doi.org/10.1016/j.gim.2023.101050.
- 99.Wimmer, M.C.; Brennenstuhl, H.; Hirsch, S.; Dötsch, L.; Unser, S.; Caro, P.; Schaaf, C.P. Hao-Fountain Syndrome: 32 Novel Patients Reveal New Insights into the Clinical Spectrum. Clin. Genet. 2024, 105, 499–509. https://doi.org/10.1111/cge.14480.
- 100.Chen, H.; Ferguson, C.J.; Mitchell, D.C.; Risch, I.; Titus, A.; Paulo, J.A.; Hwang, A.; Beck, L.K.; Lin, T.H.; Gu, W.; et al. The Hao-Fountain Syndrome Protein USP7 Regulates Neuronal Connectivity in the Brain via a Novel P53-Independent Ubiquitin Signaling Pathway. Cell Rep. 2025, 44, 115231. https://doi.org/10.1016/j.celrep.2025.115231.
- 101.Colland, F.; Formstecher, E.; Jacq, X.; Reverdy, C.; Planquette, C.; Conrath, S.; Trouplin, V.; Bianchi, J.; Aushev, V.N.; Camonis, J.; et al. Small-Molecule Inhibitor of USP7/HAUSP Ubiquitin Protease Stabilizes and Activates P53 in Cells. Mol. Cancer Ther. 2009, 8, 2286–2295. https://doi.org/10.1158/1535-7163.MCT-09-0097.
- 102.Colombo, M.; Vallese, S.; Peretto, I.; Jacq, X.; Rain, J.C.; Colland, F.; Guedat, P. Synthesis and Biological Evaluation of 9-Oxo-9H-Indeno[1,2-b]Pyrazine-2,3-Dicarbonitrile Analogues as Potential Inhibitors of Deubiquitinating Enzymes. ChemMedChem 2010, 5, 552–558. https://doi.org/10.1002/cmdc.200900409.
- 103.Chi, L.; Wang, H.; Yu, F.; Gao, C.; Dai, H.; Si, X.; Liu, L.; Wang, Z.; Zheng, J.; Ke, Y.; et al. Recent Progress of Ubiquitin-Specific-Processing Protease 7 Inhibitors. Russ. J. Bioorganic Chem. 2023, 49, 198–219. https://doi.org/10.1134/S1068162023020073.
- 104.Kategaya, L.; Di Lello, P.; Rougé, L.; Pastor, R.; Clark, K.R.; Drummond, J.; Kleinheinz, T.; Lin, E.; Upton, J.P.; Prakash, S.; et al. USP7 Small-Molecule Inhibitors Interfere with Ubiquitin Binding. Nature 2017, 550, 534–538. https://doi.org/10.1038/nature24006.
- 105.Lamberto, I.; Liu, X.; Seo, H.S.; Schauer, N.J.; Iacob, R.E.; Hu, W.; Das, D.; Mikhailova, T.; Weisberg, E.L.; Engen, J.R.; et al. Structure-Guided Development of a Potent and Selective Non-Covalent Active-Site Inhibitor of USP7. Cell Chem. Biol. 2017, 24, 1490–1500.e11. https://doi.org/10.1016/j.chembiol.2017.09.003.
- 106.Turnbull, A.P.; Ioannidis, S.; Krajewski, W.W.; Pinto-Fernandez, A.; Heride, C.; Martin, A.C.L.; Tonkin, L.M.; Townsend, E.C.; Buker, S.M.; Lancia, D.R.; et al. Molecular Basis of USP7 Inhibition by Selective Small-Molecule Inhibitors. Nature 2017, 550, 481–486. https://doi.org/10.1038/nature24451.
- 107.Leger, P.R.; Hu, D.X.; Biannic, B.; Bui, M.; Han, X.; Karbarz, E.; Maung, J.; Okano, A.; Osipov, M.; Shibuya, G.M.; et al. Discovery of Potent, Selective, and Orally Bioavailable Inhibitors of USP7 with In Vivo Antitumor Activity. J. Med. Chem. 2020, 63, 5398–5420. https://doi.org/10.1021/acs.jmedchem.0c00245.
- 108.O’Dowd, C.R.; Helm, M.D.; Rountree, J.S.S.; Flasz, J.T.; Arkoudis, E.; Miel, H.; Hewitt, P.R.; Jordan, L.; Barker, O.; Hughes, C.; et al. Identification and Structure-Guided Development of Pyrimidinone Based USP7 Inhibitors. ACS Med. Chem. Lett. 2018, 9, 238–243. https://doi.org/10.1021/acsmedchemlett.7b00512.
- 109.Di Lello, P.; Pastor, R.; Murray, J.M.; Blake, R.A.; Cohen, F.; Crawford, T.D.; Drobnick, J.; Drummond, J.; Kategaya, L.; Kleinheinz, T.; et al. Discovery of Small-Molecule Inhibitors of Ubiquitin Specific Protease 7 (USP7) Using Integrated NMR and in Silico Techniques. J. Med. Chem. 2017, 60, 10056–10070. https://doi.org/10.1021/acs.jmedchem.7b01293.
- 110.Vasas, A.; Ivanschitz, L.; Molnár, B.; Kiss, Á.; Baker, L.; Fiumana, A.; Macias, A.; Murray, J.B.; Sanders, E.; Whitehead, N.; et al. Structure-Guided Discovery of Selective USP7 Inhibitors with In Vivo Activity. J. Med. Chem. 2024, 67, 18993–19009. https://doi.org/10.1021/acs.jmedchem.4c01472.
- 111.Gavory, G.; O’Dowd, C.R.; Helm, M.D.; Flasz, J.; Arkoudis, E.; Dossang, A.; Hughes, C.; Cassidy, E.; McClelland, K.; Odrzywol, E.; et al. Discovery and Characterization of Highly Potent and Selective Allosteric USP7 Inhibitors. Nat. Chem. Biol. 2018, 14, 118–125. https://doi.org/10.1038/nchembio.2528.
- 112.Li, X.; Yang, S.; Zhang, H.; Liu, X.; Gao, Y.; Chen, Y.; Liu, L.; Wang, D.; Liang, Z.; Liu, S.; et al. Discovery of Orally Bioavailable N-Benzylpiperidinol Derivatives as Potent and Selective USP7 Inhibitors with In Vivo Antitumor Immunity Activity against Colon Cancer. J. Med. Chem. 2022, 65, 16622–16639. https://doi.org/10.1021/acs.jmedchem.2c01444.
- 113.Ohol, Y.M.; Sun, M.T.; Cutler, G.; Leger, P.R.; Hu, D.X.; Biannic, B.; Rana, P.; Cho, C.; Jacobson, S.; Wong, S.T.; et al. Novel, Selective Inhibitors of USP7 Uncover Multiple Mechanisms of Antitumor Activity In Vitro and In Vivo. Mol. Cancer Ther. 2020, 19, 1970–1980. https://doi.org/10.1158/1535-7163.MCT-20-0184.
- 114.Miao, Y.L.; Fan, F.; Cheng, Y.J.; Jia, L.; Song, S.S.; Huan, X.J.; Bao, X.B.; Ding, J.; Yu, X.; He, J.X. USP7 V517F Mutation as a Mechanism of Inhibitor Resistance. Nat. Commun. 2025, 16, 2526. https://doi.org/10.1038/s41467-025-56981-w.
- 115.Cheng, Y.J.; Zhuang, Z.; Miao, Y.L.; Song, S.S.; Bao, X.B.; Yang, C.H.; He, J.X. Identification of YCH2823 as a Novel USP7 Inhibitor for Cancer Therapy. Biochem. Pharmacol. 2024, 222, 116071. https://doi.org/10.1016/j.bcp.2024.116071.
- 116.Reverdy, C.; Conrath, S.; Lopez, R.; Planquette, C.; Atmanene, C.; Collura, V.; Harpon, J.; Battaglia, V.; Vivat, V.; Sippl, W.; et al. Discovery of Specific Inhibitors of Human USP7/HAUSP Deubiquitinating Enzyme. Chem. Biol. 2012, 19, 467–477. https://doi.org/10.1016/j.chembiol.2012.02.007.
- 117.Chauhan, D.; Tian, Z.; Nicholson, B.; Kumar, K.G.S.; Zhou, B.; Carrasco, R.; McDermott, J.L.; Leach, C.A.; Fulcinniti, M.; Kodrasov, M.P.; et al. A Small Molecule Inhibitor of Ubiquitin-Specific Protease-7 Induces Apoptosis in Multiple Myeloma Cells and Overcomes Bortezomib Resistance. Cancer Cell 2012, 22, 345–358. https://doi.org/10.1016/j.ccr.2012.08.007.
- 118.Carrà, G.; Panuzzo, C.; Torti, D.; Parvis, G.; Crivellaro, S.; Familiari, U.; Volante, M.; Morena, D.; Lingua, M.F.; Brancaccio, M.; et al. Therapeutic Inhibition of USP7-PTEN Network in Chronic Lymphocytic Leukemia: A Strategy to Overcome TP53 Mutated/Deleted Clones. Oncotarget 2017, 8, 35508–35522. https://doi.org/10.18632/oncotarget.16348.
- 119.Goldenberg, S.J.; McDermott, J.L.; Butt, T.R.; Mattern, M.R.; Nicholson, B. Strategies for the Identification of Novel Inhibitors of Deubiquitinating Enzymes. Biochem. Soc. Trans. 2008, 36, 828–832. https://doi.org/10.1042/BST0360828.
- 120.Nicholson, B.; Leach, C.A.; Goldenberg, S.J.; Francis, D.M.; Kodrasov, M.P.; Tian, X.; Shanks, J.; Sterner, D.E.; Bernal, A.; Mattern, M.R.; et al. Characterization of Ubiquitin and Ubiquitin-like-protein Isopeptidase Activities. Protein Sci. 2008, 17, 1035–1043. https://doi.org/10.1110/ps.083450408.
- 121.Altun, M.; Kramer, H.B.; Willems, L.I.; McDermott, J.L.; Leach, C.A.; Goldenberg, S.J.; Kumar, K.G.S.; Konietzny, R.; Fischer, R.; Kogan, E.; et al. Activity-Based Chemical Proteomics Accelerates Inhibitor Development for Deubiquitylating Enzymes. Chem. Biol. 2011, 18, 1401–1412. https://doi.org/10.1016/j.chembiol.2011.08.018.
- 122.Weinstock, J.; Wu, J.; Cao, P.; Kingsbury, W.D.; McDermott, J.L.; Kodrasov, M.P.; McKelvey, D.M.; Suresh Kumar, K.G.; Goldenberg, S.J.; Mattern, M.R.; et al. Selective Dual Inhibitors of the Cancer-Related Deubiquitylating Proteases USP7 and USP47. ACS Med. Chem. Lett. 2012, 3, 789–792. https://doi.org/10.1021/ml200276j.
- 123.Fan, Y.H.; Cheng, J.; Vasudevan, S.A.; Dou, J.; Zhang, H.; Patel, R.H.; Ma, I.T.; Rojas, Y.; Zhao, Y.; Yu, Y.; et al. USP7 Inhibitor P22077 Inhibits Neuroblastoma Growth via Inducing P53-Mediated Apoptosis. Cell Death Dis. 2013, 4, e867. https://doi.org/10.1038/cddis.2013.400.
- 124.Chen, C.; Song, J.; Wang, J.; Xu, C.; Chen, C.; Gu, W.; Sun, H.; Wen, X. Synthesis and Biological Evaluation of Thiazole Derivatives as Novel USP7 Inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 845–849. https://doi.org/10.1016/j.bmcl.2017.01.018.
- 125.Li, M.; Liu, S.; Chen, H.; Zhou, X.; Zhou, J.; Zhou, S.; Yuan, H.; Xu, Q.L.; Liu, J.; Cheng, K.; et al. N-Benzylpiperidinol Derivatives as Novel USP7 Inhibitors: Structure–Activity Relationships and X-Ray Crystallographic studies. Eur. J. Med. Chem. 2020, 199, 112279. https://doi.org/10.1016/j.ejmech.2020.112279.
- 126.Schauer, N.J.; Liu, X.; Magin, R.S.; Doherty, L.M.; Chan, W.C.; Ficarro, S.B.; Hu, W.; Roberts, R.M.; Iacob, R.E.; Stolte, B.; et al. Selective USP7 Inhibition Elicits Cancer Cell Killing through a P53-Dependent Mechanism. Sci. Rep. 2020, 10, 5324. https://doi.org/10.1038/s41598-020-62076-x.
- 127.Yamaguchi, M.; Miyazaki, M.; Kodrasov, M.P.; Rotinsulu, H.; Losung, F.; Mangindaan, R.E.P.; de Voogd, N.J.; Yokosawa, H.; Nicholson, B.; Tsukamoto, S. Spongiacidin C, a Pyrrole Alkaloid from the Marine Sponge Stylissa Massa, Functions as a USP7 Inhibitor. Bioorg. Med. Chem. Lett. 2013, 23, 3884–3886. https://doi.org/10.1016/j.bmcl.2013.04.066.
- 128.Jing, B.; Liu, M.; Yang, L.; Cai, H.; Chen, J.; Li, Z.; Kou, X.; Wu, Y.; Qin, D.; Zhou, L.; et al. Characterization of Naturally Occurring Pentacyclic Triterpenes as Novel Inhibitors of Deubiquitinating Protease USP7 with Anticancer Activity in Vitro. Acta Pharmacol. Sin. 2018, 39, 492–498. https://doi.org/10.1038/aps.2017.119.
- 129.Valeur, E.; Guéret, S.M.; Adihou, H.; Gopalakrishnan, R.; Lemurell, M.; Waldmann, H.; Grossmann, T.N.; Plowright, A.T. New Modalities for Challenging Targets in Drug Discovery. Angew. Chem. Int. Ed. 2017, 56, 10294–10323. https://doi.org/10.1002/anie.201611914.
- 130.Paiva, S.L.; Crews, C.M. Targeted Protein Degradation: Elements of PROTAC design. Curr. Opin. Chem. Biol. 2019, 50, 111–119. https://doi.org/10.1016/j.cbpa.2019.02.022.
- 131.Pei, Y.; Fu, J.; Shi, Y.; Zhang, M.; Luo, G.; Luo, X.; Song, N.; Mi, T.; Yang, Y.; Li, J.; et al. Discovery of a Potent and Selective Degrader for USP7. Angew Chem. Int. Ed. Engl. 2022, 61, e202204395. https://doi.org/10.1002/anie.202204395.
- 132.Murgai, A.; Sosič, I.; Gobec, M.; Lemnitzer, P.; Proj, M.; Wittenburg, S.; Voget, R.; Gütschow, M.; Krönke, J.; Steinebach, C. Targeting the Deubiquitinase USP7 for Degradation with PROTACs. Chem. Commun. 2022, 58, 8858–8861. https://doi.org/10.1039/D2CC02094G.
- 133.Chauhan, D.; Tian, Z.; Nicholson, B.; Zhou, B.; Hideshima, T.; Munshi, N.; Richardson, P.; Anderson, K.C. Deubiquitylating Enzyme USP-7, a Novel Therapeutic Target in Multiple Myeloma. Blood 2009, 114, 610. https://doi.org/10.1182/blood.V114.22.610.610.
- 134.Teleanu, D.M.; Negut, I.; Grumezescu, V.; Grumezescu, A.M.; Teleanu, R.I. Nanomaterials for Drug Delivery to the Central Nervous System. Nanomaterials 2019, 9, 371. https://doi.org/10.3390/nano9030371.
- 135.Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. https://doi.org/10.1038/s41586-021-03819-2.
- 136.Wang, L.; Wen, Z.; Liu, S.W.; Zhang, L.; Finley, C.; Lee, H.J.; Fan, H.J.S. Overview of AlphaFold2 and Breakthroughs in Overcoming Its Limitations. Comput. Biol. Med. 2024, 176, 108620. https://doi.org/10.1016/j.compbiomed.2024.108620.
- 137.Henning, N.J.; Boike, L.; Spradlin, J.N.; Ward, C.C.; Liu, G.; Zhang, E.; Belcher, B.P.; Brittain, S.M.; Hesse, M.J.; Dovala, D.; et al. Deubiquitinase-Targeting Chimeras for Targeted Protein Stabilization. Nat. Chem. Biol. 2022, 18, 412–421. https://doi.org/10.1038/s41589-022-00971-2.
- 138.Liu, J.; Hu, X.; Luo, K.; Xiong, Y.; Chen, L.; Wang, Z.; Inuzuka, H.; Qian, C.; Yu, X.; Xie, L.; et al. USP7-Based Deubiquitinase-Targeting Chimeras Stabilize AMPK. J. Am. Chem. Soc. 2024, 146, 11507–11514. https://doi.org/10.1021/jacs.4c02373.
How to Cite
Xu, X.; Zhang, N.; Shi, L. Structural Plasticity Guides Functional Versatility of USP7 in Human Diseases: Mechanistic Insights and Therapeutic Targeting. Health and Metabolism 2025, 2 (4), 6. https://doi.org/10.53941/hm.2025.100029.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


