- Shallow lakes in the sub-tropics are more prone to eutrophication and cyanotoxin pollution.
- Microcystin-LR is a commonly occurring cyanotoxin in water bodies and a potent toxin for living organisms.
- MC-LR depends on harmful algal blooms (HABs), trophic state index (TSI), and bio physicochemical variables of water bodies.
- Ecohydrological monitoring and epidemiological interventions will help in the systematic remediation of cyanotoxin pollution.
- Open Access
- Article
Enhanced Release of Cyanotoxins in Freshwater Lakes: Insights on the Causal Mechanisms and Eutrophication Dynamics in the North Bank Plains of Brahmaputra Valley, Assam, India
Author Information
Received: 17 Dec 2025 | Revised: 31 Jan 2026 | Accepted: 04 Feb 2026 | Published: 12 Feb 2026
Highlights
Abstract
Cyanotoxin pollution is a radical phenomenon involving depth of water bodies, nutrient enrichment, and dominance of cyanobacterial species. This investigation presents a novel framework for assessing cyanotoxin (MC-LR) risk analysis in the north bank plains of Brahmaputra valley, Assam, Northeast India and cyanotoxin pollution was assessed in seven sub-tropical shallow lakes in Tezpur, Sonitpur, Assam. A positive association was established between cyanotoxin pollution and lake trophic state fluctuations. MicrocystinLR (MC-LR) was quantified through a two-way analytical approach involving ELISA and HPLC methods, and the pathways of eutrophication were systematically evaluated involving CNP dynamics and limnological variables. MC-LR was detected in the seven lakes, 1.12–3.46 ppb (HPLC-method) and 1.143–19.42 ppb (ELISA-method); all lakes contained MC-LR beyond WHO permissible limits (>1 ppb). Trophic State Index (TSI) ranged between 52.05–64.59, eutrophic in four lakes and hypereutrophic in three lakes. CNP enrichment boosted algal density, chlorophyll-a, and hence MC-LR in the lakes (p < 0.05; p < 0.01). Secchi Disk Depth (SDD) had significant correlation with TSI and MC-LR potentially released by Microcystis, Dolichospermum, and Nostoc (p < 0.01); MC-LR showed substantial associations with CNP and TSI (p < 0.01). There are current or past records of MC-LR related epidemiological interventions in the study area. Therefore, this study addresses an urgent necessity to monitor cyanotoxin pollution and address the ecotoxicological concerns of harmful algal blooms (HABs) releasing cyanotoxins Northeast India lakes. Risk analysis and risk mitigation following “state of the art” cyanotoxin guidelines will facilitate systematic remediation of cyanotoxin pollution universally.
Graphical Abstract
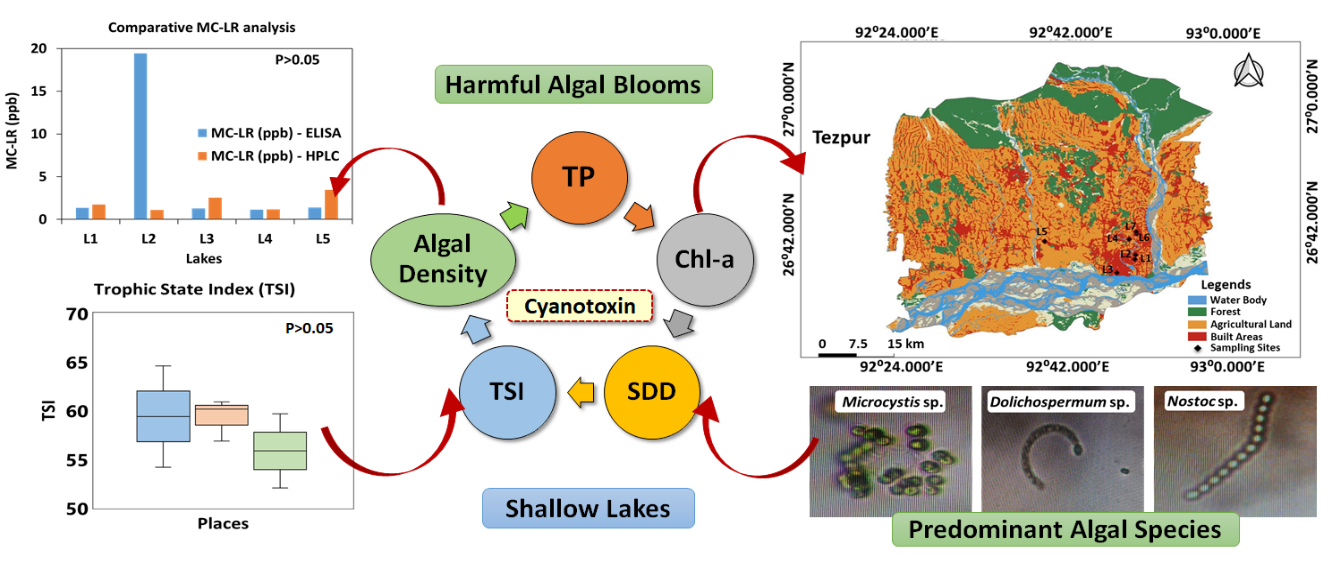
Keywords
ecohydrology | Microcystin-LR | trophic state | nutrient-enrichment | algal bloom | fresh water
References
- 1.
Bonsdorff, E. Eutrophication: Early warning signals, ecosystem-level and societal responses, and ways forward: This article belongs to Ambio’s 50th Anniversary Collection. Theme: Eutrophication. Ambio 2021, 50, 753–758. https://doi.org/10.1007/s13280-020-01432-7
- 2.
Metcalf, J.S.; Codd, G.A. Co-occurrence of cyanobacteria and cyanotoxins with other environmental health hazards: Impacts and implications. Toxins 2020, 12, 629. https://doi.org/10.3390/toxins12100629
- 3.
Paul, B.; Bhattacharya, S.S.; Gogoi, N. Ecological engineering tools for combating eutrophication. Sci. Total Environ. 2021, 762, 143171. https://doi.org/10.1016/j.scitotenv.2020.143171
- 4.
Zimba, P.V.; Moeller, P.D.; Beauchesne, K.; et al. Identification of euglenophycin, a toxin found in certain euglenoids. Toxicon 2010, 55, 100–104. https://doi.org/10.1016/j.toxicon.2009.07.004
- 5.
Du, X.; Liu, H.; Yuan, L.; et al. The diversity of cyanobacterial toxins on structural characterization, distribution and identification: A systematic review. Toxins 2029, 11, 530. https://doi.org/10.3390/toxins11090530
- 6.
Loftin, K.A.; Graham, J.L.; Hilborn, E.D.; et al. Cyanotoxins in inland lakes of the United States: Occurrence and potential recreational health risks in the EPA National Lakes Assessment. Harmful Algae 2016, 56, 77–90. https://doi.org/10.1016/j.hal.2016.04.001
- 7.
James, K.J.; Carey, B.; O’halloran, J.; et al. Shellfish toxicity: Human health implications of marine algal toxins. Epidemiol. Infect. 2010, 138, 927–940. ttps://doi.org/10.1017/S0950268810000853
- 8.
Merel, S.; Villarn, M.C.; Chung, K.; et al. Spatial and thematic distribution of research on cyanotoxins. Toxicon 2013, 76, 118–131. https://doi.org/10.1016/j.toxicon.2013.09.008
- 9.
Garita-Alvarado, C.A.; Bojorge-Garca, M.G.; Cantoral-Uriza, E.A. Cyanotoxins bioaccumulation in freshwater ecosystems in Latin America: A review. Hidrobiolo´gica 2023, 33, 353–365. http://orcid.org/0000-0001-6232-8042
- 10.
Muluye, T.; Fetahi, T.; Engdaw, F.; et al. Cyanotoxins in African waterbodies: Occurrence, adverse effects, and potential risk to animal and human health. Environ. Geochem. Health 2023, 45, 7519–7542. https://doi.org/10.1007/s10653-023-01724-3
- 11.
Abdallah, M.F.; Van Hassel, W.H.; Andjelkovic, M.; et al. Cyanotoxins and food contamination in developing countries: Review of their types, toxicity, analysis, occurrence and mitigation strategies. Toxins 2021, 13, 786. https://doi.org/10.3390/toxins13110786
- 12.
Mohan, B.; Prabha, D. Evaluation of trophic state conditions in the three urban perennial lakes of the Coimbatore district, Tamil Nadu: Based on water quality parameters and rotifer composition. HydroResearch 2024, 7, 360–371. https://doi.org/10.1016/ j.hydres.2024.06.003
- 13.
American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA: Washington DC, USA, 2017.
- 14.
Gelman, F.; Binstock, R.; Halicz, L. Application of the Walkley–Black titration for the organic carbon quantification in organic rich sedimentary rocks. Fuel 2012, 96, 608–610. https://doi.org/10.1016/j.fuel.2011.12.053
- 15.
Bowers, D.G.; Roberts, E.M.; Hoguane, A.M.; et al. Secchi disk measurements in turbid water. J. Geophys. Res. Oceans 2020, 125, e2020JC016172. https://doi.org/10.1029/2020JC016172
- 16.
Komrek, J. A polyphasic approach for the taxonomy of cyanobacteria: Principles and applications. Eur. J. Phycol. 2016, 51, 346–353. https://doi.org/10.1080/09670262.2016.1163738
- 17.
Pandit, P.R.; Fulekar, M.H.; Karuna, M.S.L. Effect of salinity stress on growth, lipid productivity, fatty acid composition, and biodiesel properties in Acutodesmus obliquus and Chlorella vulgaris. Environ. Sci. Pollut. Res. 2017, 24, 13437–13451. https://doi.org/10.1007/s11356-017-8875-y
- 18.
Rodrigues, L.H.R.; Arenzon, A.; Raya-Rodrigues, M.T.; et al. Algal density assessed by spectrophotometry: Calibration curve for Pseudokirchneriella subcapitata. J. Environ. Chem. Ecotoxicol. 2011, 3, 225–228.
- 19.
Carlson, R.E. A trophic state index for lakes 1. Limnol. Oceanogr. 1977, 22, 361–369. https://doi.org/10.4319/lo.1977.22.2.0361
- 20.
Shamsollahi, H.R.; Alimohammadi, M.; Nabizadeh, R.; et al. Monitoring of microcystin-LR concentration in water reservoir. Desalin. Water Treat. 2018, 126, 345–349. https://doi.org/10.5004/dwt.2018.22905
- 21.
Sahariah, B.; Goswami, L.; Farooqui, I.U.; et al. Solubility, hydrogeochemical impact, and health assessment of toxic metals in municipal wastes of two differently populated cities. J. Geochem. Explor. 2015, 157, 100–109. https://doi.org/10.1016/j.gexplo.2015.06.003
- 22.
Gaur, N.; Sarkar, A.; Dutta, D.; et al. Evaluation of water quality index and geochemical characteristics of surfacewater from Tawang India. Sci. Rep. 2022, 12, 11698. https://doi.org/10.1038/s41598022-14760-3
- 23.
World Health Organization. Cyanobacterial Toxins: Microcystins (No. WHO/HEP/ECH/WSH/2020.6); WHO: Geneva, Switzerland, 2020.
- 24.
Banerjee, A.; Chakrabarty, M.; Rakshit, N.; et al. Environmental factors as indicators of dissolved oxygen concentration and zooplankton abundance: Deep learning versus traditional regression approach. Ecol. Indic. 2019, 100, 99–17. https://doi.org/10.1016/j.ecolind.2018.09.051
- 25.
Liu, X.; Lee, Z.; Zhang, Y.; et al. Remote sensing of secchi depth in highly turbid lake waters and its application with MERIS data. Remote Sens. 2019, 11, 2226. https://doi.org/10.3390/rs11192226
- 26.
Feng, W.; Wang, T.; Zhu, Y.; et al. Chemical composition, sources, and ecological effect of organic phosphorus in water ecosystems: A review. Carbon Res. 2023, 2, 12. https://doi.org/10.1007/s44246023-00038-4
- 27.
Dodds, W.K. Trophic state, eutrophication and nutrient criteria in streams. Trends Ecol. Evol. 2007, 22, 669–676. https://doi.org/10.1016/j.tree.2007.07.010
- 28.
Pinheiro Menescal, M.T.A.; Almeida, E.D.S.; Sales, E.A.; et al. Identification of cyanobacteria and its potential toxins in the Joanes I Reservoir, Bahia, Brazil. Toxins 2023, 15, 51. https://doi.org/10.3390/toxins15010051
- 29.
Sahoo, D.; Tran, N.K.N.; Nguyen, T.G.H.; et al. Co-occurrence of cyanotoxins and phycotoxins in Tam Giang–Cau Hai Lagoon. Limnol. Rev. 2024, 24, 335–353. https://doi.org/10.3390/limnolrev24030020
- 30.
Lin, J.L.; Karangan, A.; Huang, Y.M.; et al. Eutrophication factor analysis using Carlson trophic state index (CTSI) towards non-algal impact reservoirs in Taiwan. Sustain. Environ. Res. 2022, 32, 25. https://doi.org/10.1186/s42834-022-00134-x
- 31.
Huszar, V.L.; Caraco, N.F.; Roland, F.; et al. Nutrient–chlorophyll relationships in tropical–subtropical lakes: Do temperate models fit? Biogeochemistry 2006, 79, 239–250. https://doi.org/10.1007/s10533006-9007-9
- 32.
Cunha, D.G.F.; do Carmo Calijuri, M.; Lamparelli, M.C. A trophic state index for tropical/subtropical reservoirs (TSItsr). Ecol. Eng. 2013, 60, 126–134. https://doi.org/10.1016/j.ecoleng.2013.07.058
- 33.
Nowicka-Krawczyk, P.; Z˙elazna-Wieczorek, J.; Skrobek, I.; et al. Persistent cyanobacteria blooms in artificial water bodies—An effect of environmental conditions or the result of anthropogenic change. Int. J. Environ. Res. Public Health 2022, 19, 6990. https://doi.org/10.3390/ijerph19126990
- 34.
Kemp, A.; John, J. Microcystins associated with microcystis dominated blooms in the southwest wetlands, Western Australia. Environ. Toxicol. 2006, 21, 125–130. https://doi.org/10.1002/tox.20164
- 35.
Otieno, P.O.; Owuor, P.O.; Lalah, J.O.; et al. Comparative evaluation of ELISA kit and HPLC DAD for the determination of chlorpyrifos ethyl residues in water and sediments. Talanta 2013, 117, 250–257. https://doi.org/10.1016/j.talanta.2013.09.014
- 36.
Hollister, J.W.; Kreakie, B.J. Associations between chlorophyll a and various microcystin health advisory concentrations. F1000Research. 2016, 5, 151. https://doi.org/10.12688/f1000research.7955.2
- 37.
Farrer, D.; Counter, M.; Hillwig, R.; et al. Health-based cyanotoxin guideline values allow for cyanotoxin-based monitoring and efficient public health response to cyanobacterial blooms. Toxins. 2015, 7, 457–477. https://doi.org/10.3390/toxins7020457
- 38.
Kelly, N.E.; Javed, A.; Shimoda, Y.; et al. A Bayesian risk assessment framework for microcystin violations of drinking water and recreational standards in the Bay of Quinte, Lake Ontario, Canada. Water Res. 2019, 162, 288–301. https://doi.org/10.1016/j.watres.2019.06.005
- 39.
MacKeigan, P.W.; Taranu, Z.E.; Pick, F.R.; et al. Both biotic and abiotic predictors explain significant variation in cyanobacteria biomass across lakes from temperate to subarctic zones. Limnol. Oceanogr. 2023, 68, 1360–1375. https://doi.org/10.1002/lno.12352

This work is licensed under a Creative Commons Attribution 4.0 International License.


